Physics For Civil Engineering: Unit IV: New Engineering Materials
Non Crystalline Ceramics
These are usually regarded super, cooled liquids. Their molecules are not arranged in regular geometric shapes.
NON CRYSTALLINE CERAMICS
These are usually regarded super, cooled
liquids. Their molecules are not arranged in regular geometric shapes. e.g.
amorphous or fused SiO2 has each Si bonded to four O and each O is
bonded to two Jairo (S)
This type of ceramics is used for
mirrors, optical lenses, reinforcement fibres for GRP and optical fibres for
data transmission. anoites llama
Silicates
and Silica
Silicates
are composed of silicon and oxygen, which are abundantly available in the
earth's crust. For example, rocks, soils and clay come under the classification
of silicates.
A unit cell of silicate is a tetrahedron
on which each atom of silicon is bounded to four atoms of oxygen as shown in
fig 4.18. over of abnuo 02
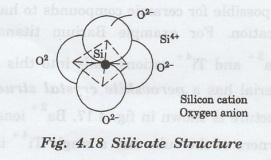
Oxygen
atoms are located on the edges of a tetrahedron structure and silicon atoms are
located at the centre.
This basic unit of silicate is treated as negatively charged. There is a covalent bond between Si and O, i.e. Si-O.
Silica
Silica
(SiO2) is the simple form of silicate. This is a three-dimensional network
of tetrahedron where every corner oxygen atom is shared by adjacent
tetrahedral. This material becomes electrically neutral but al but
electronically stable.
Under this arrangement, the ratio of Si
to O becomes 1:2 as
given by chemical formula, SiO2.
There
are three polymorphic forms of silica: (1) quartz, (2) cristobalite and (3)
tridymite. Silica is used in the manufacture of different varieties of glasses.
Structure of glasses
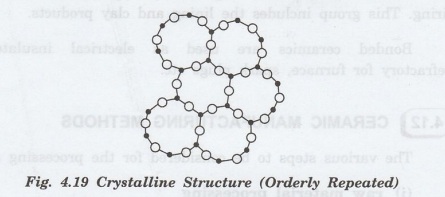
Generally,
solids have three-dimensional periodic structures as shown in figure 4.19. This
is a crystalline structure.
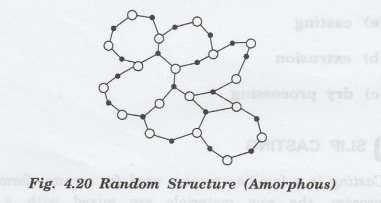
The
materials, which do not have three-dimensional structures, but random structure
as shown in figure 4.20 are said to be amorphous or glassy.
Many
metal alloys, oxide compounds and non-oxide compounds form glassy structure.
Fused silica or vitreous silica has high degree of atomic randomness.
Similarly, oxide as B2O3 and GeO2 may also
form glassy structure.
The
glasses that are used for containers and windows are silica glasses in which
oxides such as CaO and Na2O are added.
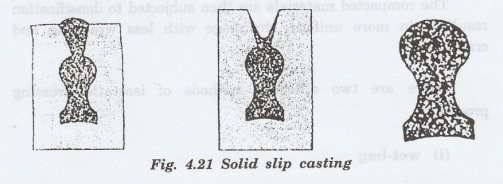
The
thickness of solid layer depends on the length of time in mold. This process is
continued until the entire mold cavity becomes solid. This process is known as
slip casting and the various stages are shown in Fig. 4.21.
Advantages:
The
main advantage of slip casting is the ability to form intricate shapes at
relatively low cost.
The
complex ceramic shapes which are produced using slip casting include turbine
engine rotors, automobile wings, etc.
Physics For Civil Engineering: Unit IV: New Engineering Materials : Tag: : - Non Crystalline Ceramics
Related Topics
Related Subjects
Physics for Civil Engineering
PH3201 2021 Regulation | 2nd Semester Civil Dept 2021 Regulation
