Physics For Civil Engineering: Unit IV: New Engineering Materials
Metallic Glasses
Preparation, Principle, Types, Properties, Applications
In the year 1970, scientists discovered metallic glasses which are a new class of materials. Their important characteristic is non-crystalline structure.
METALLIC GLASSES
In the year 1970, scientists discovered
metallic glasses which are a new class of materials. Their important
characteristic is non-crystalline
structure. But, the normal metal alloys have crystalline structure. They are also called as amorphous metals.
Metallic glass = Amorphous metal
Generally, glass is an amorphous,
brittle and transparent solid. We know that the metals are malleable, ductile
and exhibit crystalline properties. The
metallic glasses have the properties of both metals and glasses.
It is found that the metallic glasses
are strong, ductile, malleable, opaque and brittle. They have good magnetic
properties and high corrosion resistance. leiretam
Metallic glasses are usually prepared by
cooling a metallic liquid (which has a disordered structure) so rapidly such that there is no enough
time for ordered crystalline structure to develop.
During
the solidification, there is essentially no change in spatial atomic
configuration. Thus, a glass may be
considered as a solid frozen in liquid structure.
Glass
transition temperature
Temperature
at which transition from liquid to solid occurs is known as glass transition
temperature (Tg).
The
change of state from molten liquid to metallic glasses is shown in fig. 4.4.
Case (1): In fig. 4.4, the curve ABDE shows
the change of state from molten liquid to crystalline solid at the temperature
called melting point temperature (Tm).
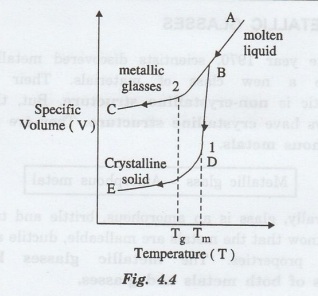
This occurs due to normal rate of
cooling with the decrease in its specific volume. Thus it forms a normal
crystalline material.
Case
(2):
The curve he curve ABC shows the the change of state from a molten liquid to
metallic glasses due to rapid cooling without decrease in its specific volume
at the temperature called glass
transition temperature (Tg). Thus, it forms materials with
crystalline property.
The glass transition temperature for the
metallic glasses is about 20°C to 300°C instead of several hundred degrees as
in glasses. Upon heating, the metallic glasses show a reversible glass liquid
transition at Tg
Preparation of Metallic Glasses
Principle
The
principle used in making metallic glasses is extreme rapid cooling of the
molten metal alloy. This technique is called as rapid quenching.
Melt
spinning systems
A
melt spinner consists of a copper roller over which a refractory tube with fine
nozzle is placed. The refractory tube is provided with induction heater as
shown in fig. 4.5.
The
metal alloy is melted by induction heating under inert gas atmosphere (helium
or argon). The properly super heated molten alloy is ejected through the fine
nozzle at the bottom of the refractory tube.
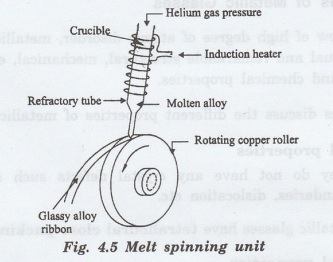
The
molten alloy falls on the copper roller which is rotated at high speed. Thus,
the alloy is suddenly cooled to form metallic glass. In this method a
continuous ribbon of metallic glass can be obtained.
Types of Metallic glasses
Metallic
glasses are classified into two types:
(i)
Metal - Metal metallic glasses
They
are combination of metals
Example:
Nickel
(Ni) - Niobium (Nb)
Magnesium
(Mg) - Zinc (Zn)
Copper
(Cu) - Zirconium (Zr)
(ii)
Metal - Metalloid metallic glasses
These
are combinations of metals and metalloids.
Example:
Metals:
Metalloids
Fe, Co, Ni B,Si C, P
Properties
of Metallic Glasses
In view of high degree of atomic
disorder, metallic glasses show unusual and remarkable structural, mechanical,
electrical, magnetic and chemical properties.
Let
us discuss the different properties of metallic glasses.
Structural
properties
•
They do not have any crystal defects such as grain boundaries, dislocation etc.
• Metallic glasses have tetrahedral
close packing (TCP).
Mechanical
properties
• Metallic glasses have extremely high
strength, due to the absence of point defects and dislocation.
•
They have high elasticity.
They
are highly ductile.
• Metallic glasses are not work-harden
but they are work-soften. (work hardening is a process of hardening a material
by compressing it)
Electrical
properties
• Electrical resistivity of metallic
glasses is high and it does not vary much with temperature.
•
Due to high resistivity, the eddy current loss is very small.
•
The
temperature coefficient is zero or negative.
Magnetic
properties
• Metallic glasses have both soft and
hard magnetic properties.
• They are magnetically soft due to their maximum
permeabilities. Thus, they can be magnetised and demagnetised very easily.
•
They exhibit high saturation magnetisation.
•
They have less core losses.
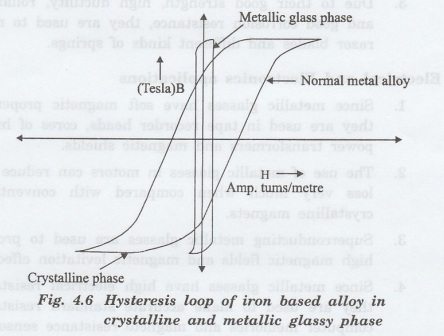
•
Most magnetically soft metallic glasses have very narrow hysteresis loop with
same crystal composition. This is shown in fig 4.6.
Chemical
properties
•
They
are highly resistant to corrosion due to
random 918 yedi nedW
•
They are highly reactive and stable.
•
They can act as a catalyst. The amorphous state is more floa active than the
crystalline state from the catalytic point of view.
Applications of Metallic Glasses
Metallic
glasses are also called as met glasses. They have found wide applications in
different fields.
Structural
applications
1.
They possess high physical and tensile strength. They are superior to common
steels and thus they are very useful as reinforcing elements in concrete,
plastic and rubber.
2.
Strong ribbons of metallic glasses are used for simple filament winding to
reinforce pressure vessels and to construct large fly wheels for energy
storage.
3.
Due to their good strength, high ductility, rollability and good corrosion
resistance, they are used to make razor blades and different kinds of springs.
Electrical
and Electronics applications
1.
Since metallic glasses have soft magnetic properties, they are used in tape
recorder heads, cores of high power transformers and magnetic shields.
2. The use of metallic glasses in motors
can reduce core loss very much when compared with conventional crystalline
magnets.
3.
Superconducting metallic glasses are used to produce high magnetic fields and
magnetic levitation effect.
4.
Since metallic glasses have high electrical resistance, they are used to make
accurate standard resistance, computer memories and magneto resistance sensors.
5. Metallic glasses as transformer
core material
Metallic
glasses have excellent magnetic properties. When they are used as transformer
core, they give maximum magnetic flux linkage between primary and secondary
coils and thus reduce flux leakage losses.
In
view of their features like small thickness, iniog sidy smaller area, light weight,
high resistivity, soft magnetic property and negligible hysteresis and eddy
current loss, metallic glasses are considered as core materials in different
frequency transformers.
Nuclear
reactor engineering applications
1.
The magnetic properties of metallic glasses are not affected by irradiation.
So, they are useful in preparing containers for nuclear waste disposal and
magnets for fusion reactors.
2. Chromium and phosphorous based (iron
chromium, phosphorous - carbon alloys) metallic glasses have high corrosion
resistances. Hence they are used in inner surfaces of reactor vessels.
Bio-medical
Industries applications
1. Due to their high resistance to
corrosion, metallic glasses are ideal materials for making surgical
instruments.
2.
They are used as prosthetic
materials for implantation in human body.
Physics For Civil Engineering: Unit IV: New Engineering Materials : Tag: : Preparation, Principle, Types, Properties, Applications - Metallic Glasses
Related Topics
Related Subjects
Physics for Civil Engineering
PH3201 2021 Regulation | 2nd Semester Civil Dept 2021 Regulation
