Physics For Civil Engineering: Unit IV: New Engineering Materials
Crystalline Ceramics
These have simple crystal structure, such as aluminium oxide (corundum), magnesium oxide, silicon carbide. Most of the oxides can be considered packing of oxygen ions with the cations occupying the tetrahedral and / or octahedral sites in the structure.
CRYSTALLINE CERAMICS
These have simple crystal structure,
such as aluminium oxide (corundum), magnesium oxide, silicon carbide. Most of
the oxides can be considered packing of oxygen ions with the cations occupying
the tetrahedral and / or octahedral sites in the structure.
Magnesium
oxide is used in refractory furnace lining for steel making. Silicon carbide is
used for cutting tools.
The
crystal structure of ceramic is, more complex, since atom of different size and
electronic configuration are assembled together.
Common
crystal structures found in crystalline ceramics particularly those of the
oxide type are briefly described below:
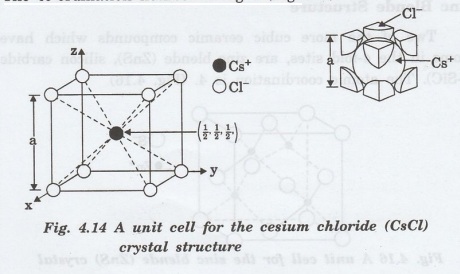
Cesium Chloride Structure
It
is possible for ceramic compounds to have simple cubic structure that are not
found among metals. Cesium chloride is a prototype for this case.
In
this structure, chlorine ions are arranged in a simple cubic structure and all
interstices are occupied by cesium ions. The co-ordination number is eight
(Fig.4.14).
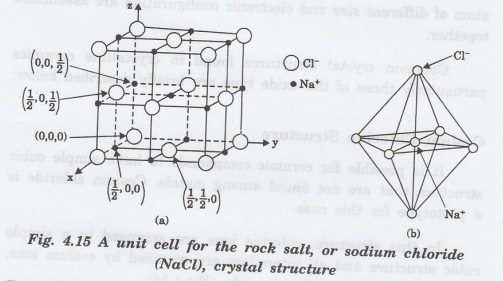
Rock Salt Structure
Most
of the oxides and halides crystallize in the closed packed cubic structure
similar to that of a rock salt (sodium chloride). The structure can be
considered as consisting of the FCC anions with smaller cations filling all
available interstitial positions.
Here, each metal atom is surrounded by
six non-metallic atoms and vice versa (Fig. 4.15). Thus, atomic coordination
(CN) is 6. Other examples are MgO, CaO, BaO, CdO, MnO, FeO, CeO and NiO.
Zinc Blende Structure
Two
of the more cubic ceramic compounds which have atoms in the 4-fold sites, are
zinc blende (ZnS), silicon carbide (B-SiC). The atomic coordination is 4. (Fig.
4.16)
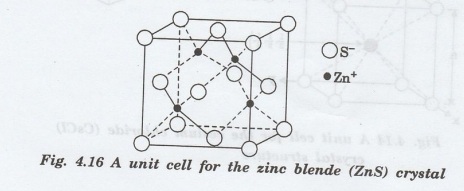
•
Each type of atom form an FCC structure of its own.
•
Only half of the available tetrahedral interstices are filled with the small
cations.
•
The structure is the same as the diamond cubic except that alternate atoms are
of different elements.
This structure also includes cadmium
sulphide (CdS) and aluminium phosphide (AIP).
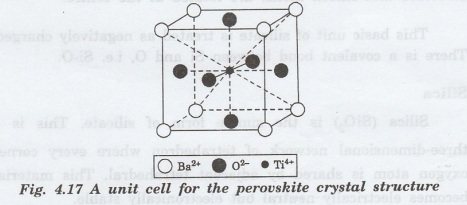
Perovskite Crystal Structure
It
is also possible for ceramic compounds to have more than one type of cation.
For example Barium titanate (BaTiO3), having both Ba2+ and
Ti4+ cations, falls into this classification.
This
material has a perovskite crystal
structure. A unit cell of this structure is shown in fig. 4.17. Ba2+
ions are situated at all eight corners of the cube and a single Ti 4+is
at the cube center. The 02- ions located at the center of each of
the six faces of the unit cell.
Physics For Civil Engineering: Unit IV: New Engineering Materials : Tag: : - Crystalline Ceramics
Related Topics
Related Subjects
Physics for Civil Engineering
PH3201 2021 Regulation | 2nd Semester Civil Dept 2021 Regulation
