Physics For Civil Engineering: Unit I: Thermal Application
Principles of Heat Transfer
Heat transfer is a discipline of thermal engineering that concerns with exchange of thermal energy or heat between physical systems.
PRINCIPLES OF HEAT TRANSFER
Heat transfer is a discipline of thermal
engineering that concerns with exchange of thermal energy or heat between
physical systems.
When the temperature of a body
increases, the energy supplied to the body is being stored in the form of
thermal or heat energy.
In the normal process, the transmission
of heat takes place from a region of higher temperature to a region of lower
temperature. There are three modes of transmission of heat (Fig 1.1).
They
are
1. Conduction
2. Convection
3. Radiation

Thermal Conduction, Convection and Radiation
Thermal Conduction:
It is the process in which heat is
transferred from one point to another through the substance without the actual
motion of the particles.
Thermal Convection:
It is the process in which heat is
transmitted from one place to another by the actual motion of the heated
particles.
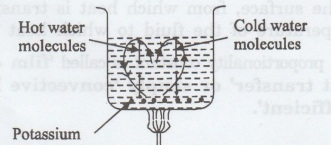
Example:
Let
us consider a beaker of water heated by a flame as shown in figure 1.2. The
water in the central portion at the bottom of beaker gets heated first.
It rises up and the water from the top
comes down along the sides to get heated. This upward and downward motion can
be made visible by placing a crystal of potassium permanganate at the bottom of
the beaker.
The hot air furnace, hot water heating
system and the flow of blood in the body are examples of convection.
This takes places in case of liquids or
gases only.
Types
of convection
There are two types of convection.
(i) Natural convection
(ii) Forced convection
Natural convection
If the convection is induced by density
differences, resulting from temperature differences within the fluid, then it
is termed as natural convection.
Forced convection
If the fluid motion is caused by
external mechanical means, e.g. by a pump, fan, etc., the process is known
'forced convection'.
Amount of heat transfer
The amount of heat transfer by
convection is calculated on the basis of the Newton's law of cooling.
Amount of heat transferred
Q = h A
where A
- area of the surface from which heat is transferred
∆t temperature difference between the
temperature of the surface, from which heat is transferred and the temperature
of the fluid to which heat is transferred.
h- proportionality constant is called 'film coefficient of heat transfer' or
simply 'convective heat transfer coefficient'.
Thermal Radiation
It is the process in which heat is
transmitted from one place to the other directly, without any material medium.
Example:
The heat radiation from the sun reaches us with an enormous velocity of light
without any intervening medium as shown in fig. 1.3.

Though the sun is millions of miles away
from the earth and there is no material medium for the greater part of the
distance, the heat reaches us with the velocity of light.
Thus, heat radiations can pass through
vacuum. The properties of heat radiations are similar to light radiation.
Properties
of Thermal Radiation
• They travel through vacuum just like
light.
• Like light, they travel in straight
lines.
• They travel with the same velocity as
light. aud eluselor re
• Radiant energy follows the law of
inverse square as light.
• They exhibit the phenomenon of
reflection and refraction as light.
Heat
Conductions in Solids
It is a well known fact that heat is
conducted through the material of a body. E
The heat is transmitted from a body of
higher temperature to that of lower temperature.
As an example, when a metal rod is
heated at one end, heat gradually flows along the length of the rod and other
end of the rod also becomes hot after some time. (Fig. 1.4)

This shows that heat has travelled
through the molelcules of the rod from one end to other. The molecules in the
rod remain fixed in their mean positions.
On heating, the energy of molecules
increases and they start vibrating more about their mean positions. They
collide with the neighbouring molecules. Because of this collision, the
neighbouring molecules are set into vibration.
Each molecule thus transfers some of
heat it receives from its predecessor to its successor. Thus, the transmission of heat takes place by molecular vibrations in
the case of conduction.
Definition
It is the process of transmission of
heat from one point to another point through a solid substance (or some medium)
without the actual motion of the particles (molecules or atoms) of the
subtance.
Note
Heat conduction always requires some material medium. The material medium must be solid. As it requires material medium, the heat conduction process never takes place in vacuum
Physics For Civil Engineering: Unit I: Thermal Application : Tag: : - Principles of Heat Transfer
Related Topics
Related Subjects
Physics for Civil Engineering
PH3201 2021 Regulation | 2nd Semester Civil Dept 2021 Regulation
