Water Supply And Wastewater Engineering: Unit II: Water Treatment
Two Marks Questions with Answers
Water Treatment | Water Supply and Wastewater Engineering
Water Supply And Wastewater Engineering: Unit II: Water Treatment: Two Marks Questions And Answers
TWO
MARK QUESTION AND ANSWERS
1. What are the main objectives of
treating water?
(i) To remove colour, dissolved gases and murkiness of water
(ii) To remove objectionable tastes and odour.
(iii) To remove the disease producing micro-organism so that
water is safe for drinking purposes
(iv) To remove hardness of water
(v) To make water
suitable for industrial purposes such as brewing, dyeing and steam generation
2. Differentiate between unit
operations and unit process in context of water treatment?
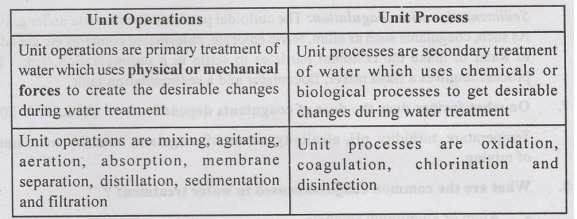
Unit Operations
Unit operations are primary treatment of water which uses
physical or mechanical forces to create the desirable changes
Unit operations are mixing, agitating, aeration, absorption,
membrane separation, distillation, sedimentation and filtration
Unit Processi
Unit processes are secondary treatment of water which uses
chemicals or biological processes to get desirable changes during water
treatment
Unit processes es are oxidation, coagulation, chlorination
disinfection
3. What are the various unit
operations and unit processes used in the treatment of water?
Screening, Plain Sedimentation, Sedimentation with coagulation,
Filtration, Disinfection, Aeration, Water Softening etc.
4. What are the factors influencing
the settling of a particle?
• Horizontal flow velocity of water
• Viscosity of water
• Size and shape of particle
• Specific gravity of the particle
• Temperature of water
• Short circuiting of flow
• Scour velocity
5. State stokes equation for
finding settling velocity of particles?
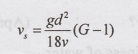
V1 = settling velocity of particle
g = acceleration due to gravity
d = diameter of the particle
G = specific gravity of the particle
v = kinematic viscosity of particle
6. Differentiate between Plain
Sedimentation and Sedimentation with coagulation
Plain Sedimentation: When
water is detained in a tank, the large discrete suspende yot particles settle
under gravity which is known as plain sedimentation. This require ($10 only a
sedimentation tank.
Sedimentation with coagulation: The colloidal particles do not settle under gravity As such, coagulants
such as alum, ferric chloride, chlorinated copperas etc are adde to water to
make the colloidal particles to settle in a sedimentation tank. Thi process
requires a flash mixer, flocculator and a sedimentation tank.
7. On what factors does the dose of
coagulants depend?
Temperature, turbidity, pH, alkalinity, nature of coagulant,
intensity and duratio br of mixing.
8. What are the common coagulants
used in water treatment?
• Alum or aluminium sulphate
• Chlorinated copperas
• Iron salts - ferrous sulphate, ferric chloride, ferric
sulphate no.
• Sodium aluminate
9. What is the significance of
velocity gradient in flash mixer?
Velocity gradient (G) determines the mixing efficiency and power
consumption a flash mixer. It also controls the floc formation in flocculators.
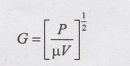
10. Define: Detention time and
surface over flow rate for a sedimentation tank?
Detention time is theoretical time taken by a particle of water to pass betwee
entry and exit of the settling tank. t = volume of tank / rate of flow
Surface loading rate
or surface over flow rate
The quantity of water passing per hour per unit horizontal area
is known as the ove flow rate or surface loading.
SOR or SLR = Discharge/Surface Area
11. What is coagulation?
The process of addition and mixing of chemicals (coagulants) in
water to aid in th settling of colloidal and fine suspended particles is called
coagulation.
12. Define Flocculation.
The process of floc formation by the aggregation of chemical
precipitate and colloid is known as flocculation.
13. Define filtration. What are the
different types of filters?
The process of passing the water through the beds of granular
materials is known a filtration.
Types: Slow sand filters, Rapid sand filters, Pressure filters
etc
14. What is schmutzdecke layer or
dirty skin?
After few weeks of commissioning the sand filters, a layer of
algae, bacteria protozoa, suspended particles and partly decomposed organic
matter is formed o the surface of sand, which is called the dirty skin or
schmutzdecke layer. This laye helps in further absorbing organic matter from
water.
15. What is the theory of
filtration?
• Mechanical straining
• Sedimentation
• Biological metabolism
• Electrolytic changes
16. List out advantages of rapid
sand filter?
• High filtration rate 3000 to 6000 litres/m2/hr
• Occupies less area
• Less initial cost
• Donoldo boilggs
17. What are the operational
problems in sand filters?
Formation of mud balls: Mud from atmosphere may accumulate on
the sand surface. It is broken by mechanical rakes or by washing with water
under pressure.
Cracking or clogging of filters: The fine sand in the top layers
may shrink causing ow shrinkage cracks.
Air binding: The dissolved air and gases from water, occupy the
void space of filter media. This can be controlled by preventing any algal
growth on filters and by Tavo preventing super-saturation of water.
18. What are the advantages and
disadvantages of pressure filters?
Advantages: Compact, automatic operation, requires small area for installation,
very high rate of filtration 6000 to 15000 litres per hour per square meter.
Disadvantages: Treatment cost is high, efficiency of bacteria removal is low.
19. Differentiate between
sterilisation and disinfection?

20. List the different methods of
disinfection. Enumerate the mechanism o disinfection process?
Mechanism of disinfection may be chemical or physical which are
tabulated below
Physical: Boiling, Ultra-violet radiation.
Chemical: Chlorination, Bromine and Iodine, Potassium permanganate, Ozon
Excess Lime.
21. What is break point
chlorination?
The chlorine added to water is utilized in the oxidation of
organic matter and killin of bacteria. It reacts with the ammonia to form
chloramines. During the initi phase of chorine addition, the residual chlorine
or free chlorine will be less than the applied chlorine. But further dosage of
cholorine beyond a certain value. Will equally appear as free residual chlorine. This dosage of chlorine is
called as break point chlorination. Residual chlorine limit is 0.2 to 0.3 mg/l
22. What are the advantages of
chlorine as disinfectant?
• Kills germs and disease causing bacteria effectively
• Cheap and easily available
• Can be stored for long periods
• Prevention of algal growths
• Taste and odour control
23. What are the tests to be done
to find the residual chlorine in water?
• Orthotolidine test
• D.P.D test
• Chlorotex test
• Starch Iodide test
24. How to manage the residue in
water treatment plant?
Land filling, horticulture use, disposal to waste water
injection, regeneration of coagulants, incineration.
25. Define aeration.
Aeration is the process of gas transfer between water and air.
26. State the objectives of
aeration process in water treatment?
• To remove dissolved gases, such as carbon dioxide, hydrogen
sulfide
• To remove iron and manganese.
• To remove taste and odour.
• To increase the DO of water.
27. Mention the types of aerators
used in water treatment?
(a) Spray nozzles
(b) Cascade aerators
(c) Air diffusers
(d) Trickling bed aerators
28. List the pollutants that get
removed in an aerator?
Carbon dioxide, Hydrogen sulfide (rotten-egg odour), Methane
(flammable), Iron (stains clothes and fixtures), Manganese (black stains),
Volatile organic chemicals, taste and odour
29. How do you remove iron and
manganese from water?
List out the unit process applied
to remove iron and manganese from water?
Iron and manganese from water can be removed by the following
methods:
(a) Aeration
(b) Manganeze Zeolite
Addition of lime, chlorine and potassium permanganate
30. Define hardness of water? What
are the types of hardness present in water?
It is a property of water, which prevents the lathering of soap.
Hardness is of two types: Temporary Hardness and Permanent Hardness
31. Differentiate between Temporary
Hardness and Permanent Hardness?
Temporary Hardness
This is due to the presence of carbonate and bicarbonate of
Calcium and
This is also called as Carbonate This is also called as
Non-Carbonate Hardness
It can be removed easily
by boiling and adding lime
Permanent Hardness
This is due to the presence of sulphates, chlorides and nitrates
of Calcium and Magnesium
This is also called as Non- Carbonate Hardness
Its removal requires
special treatment like zeolite or lime-soda process.
32. Describe about the term water
softening?
Reduction or removal of hardness from water is known as water
softening. Temporary hardness is removed by boiling and addition of lime.
Permanent hardness is removed by: Lime-soda process, base
exchange or zeolite process, and demineralization process.
33. List out any four effects of
hardness in water?
• Causes more consumption of soap in laundry work.
• Affects dyeing of textiles
• Causes difficulties in paper, canning, ice and rayon industry
• Causes choking and clogging of pipes
• Causes scaling in boilers and heaters
• Makes food tasteless, tough or rubbery
34. What are the methods of
removing hardness?
• Boiling
• Addition of lime.
• Lime-soda process
•Base exchange or Zeolite process
35. What is Zeolite process?
Silicates of aluminium and sodium compounds, which exchange
calcium and magnesium ions for sodium ions is known as zeolite process.
Zeolites are used in the water softening process to remove permanent hardness
from water.
36. What are the advantages of
Zeolite process?
No sludge is formed, hence problem of sludge disposal does not
arise.
It can be operated easily and no skilled supervision is
required.
Hardness of water reduces to zero and hence used for boiler and
textile industries. Economical process
37. What is demineralization?
It is the removal or reduction of minerals in water to make it
suitable for industrial and domestic purposes. It involves cation exchange
process followed by anion exchange process.
38. How do you regenerate softener?
Water softeners can be regenerated by treating with 5-10%
solution chloride (Brine solution)
CaZ + 2NaCl = Na2Z + CaCl2
Mg + 2NaCl = Na2Z + MgCl2
39. Define Fluoridation?
If the fluoride concentration in water is less than 1 mg/l, excess
fluoride is added to water which is called as fluoridation.
40. Name the methods of
deflouridation?
• Prashanthi technique using adsorption by activated alumina
• Ion exchange adsorption method
• Nalgonda technique
• Reverse osmosis process
41. What is the maximum permissible
limit of fluoride in drinking water?
Acceptable limit of flouride in drinking water is 1 mg/l
42. Define the term fluorosis.
Disease caused by intake of excess fluoride affecting the bone
and teeth is known as fluorosis.
43. Water Supply and Waste Water
Engineering
Dental fluorosis: discolouration and pitting of teeth
Skeletal fluorosis: severe bone deformation
Non-skeletal
fluorosis: gastrointestinal problems,
neurological problems
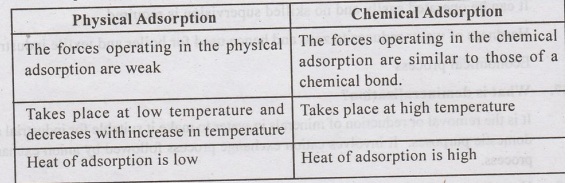
44. Distinguish between physical a dsorption
and chemical adsorption?
45. Write down the principle of
desalination of water?
Desalination is the process of removing dissolved salts from
water, thus producin fresh water from seawater or brackish water. Salts are
present in water as Na+ catio and Cl- anion. These ions are separated either by
oppositely charged electrodes o ion selective membranes.
46. Mention any four methods of
desalination process?
a. Evaporation and Distillation
b. Electrodialysis
c. Reverse osmosis
d. Freezing process
e. Solar distillation method
47. What is Reverse Osmosis?
The natural osmotic pressure is opposed by exerting an external
pressure on t side containing the salt solution which forces pure water from
the salt solution [S10 move across a semi-permeable membrane towards the side
containing water. Th process is called as reverse osmosis.
Water Supply And Wastewater Engineering: Unit II: Water Treatment : Tag: : Water Treatment | Water Supply and Wastewater Engineering - Two Marks Questions with Answers
Related Topics
Related Subjects
Water Supply and Wastewater Engineering
CE3303 3rd Semester Civil Dept 2021 Regulation | Tag: 3rd Semester Civil Dept 2021 Regulation
