Water Supply And Wastewater Engineering: Unit II: Water Treatment
Water Softening
Objectives, Methods, Working Principle, Advantages, Disadvantages
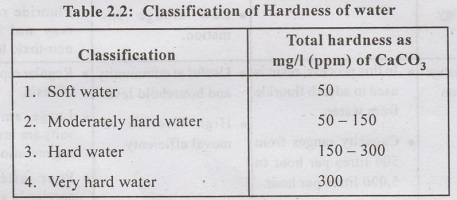
Temporary/Carbonate Hardness - is caused by the carbonates and bicarbonates of calcium and magnessium. Temporary hardness can be easily removed by boiling or adding lime.
SOFTENING
•
The reduction or removal of hardness from water is called water softening.
•
Hard water causes the following problems :-
(i)
It causes more consumption of soap in laundry work.
(ii)
It causes modification of colours and affects the dyeing industries.
(iii)
It causes serious difficulties in the manufacturing process such as paper
making, al grind ice manufacture, Rayon industry etc.
(iv)
It causes choking and clogging of plumbing fixtures.
(v)
It causes scale formation in boilers and hot water heating system.
(vi)
It makes the food tasteless, tough or rubbery.
Objectives of water
softening are:
(i)
To reduce the soap consumption of water
(ii)
Improve the food taste
(iii)
To reduce the maintenance of plumbing fixtures
(iv)
Prevent scaling of boilers
(v)
Improves the efficiency of manufacturing and dying processes.
(vi)
Improves the efficiency of filtration etc.
•
Temporary/Carbonate Hardness - is caused by the carbonates and bicarbonates of
calcium and magnessium. Temporary hardness can be easily removed by boiling or
adding lime.
•
Permanent / Non-carbonate Hardness is caused by the sulphates, chlorides,
nitrates of Calcium and Magnesium. This cannot be easily removed and requires
special methods of water softening such as lime-soda process, Zeolite process
or Demineralisation.
Methods of removing Temporary
Hardness :-
1. Boiling:
•
Calcium carbonate is not readily soluble in water.
•
It may exist as Calcium bicarbonate in water, which easily dissolves in
water containing Carbon dioxide.
•
When the water is boiled, CO2 is released, leading to precipitation of CaCO3
which can be removed by sedimentation process in a settling tank.
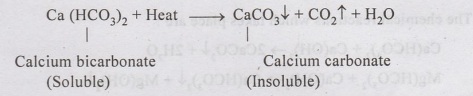
•
This method cannot be used for Magnesium carbonate and Magnesium bicarbonate,
since MgCO3 is soluble in water.
Limitation:
•
Boiling does not remove temporary hardness caused by magnesium.
•
Boiling is unfeasible and uneconomical for public water supplies.
2. Addition of Lime
Lime
(CaO), generally hydrated lime [Ca(OH)2] is added to the water. The following
reactions take place :

The
calcium carbonate and magnesium hydroxide are precipitated which can be removed
in the sedimentation tank.
Methods of removing Permanent
Hardness:
The
different methods of removing permanent hardness are :
(1)
Lime - soda process
(2)
Base - Exchange process/ Zeolite process
(3)
Demineralisation process
1. Lime - Soda
process:
•
In this process, lime [Ca(OH)2] and soda ash [Na2 CO3]
are added to hard water; which react with calcium and magnesium salts, to form
insoluble precipitates of calcium carbonate and magnesium hydroxide.
•
These precipiates can be sedimented out in a sedimentation tank.
•
The chemical reactions which takes place are :
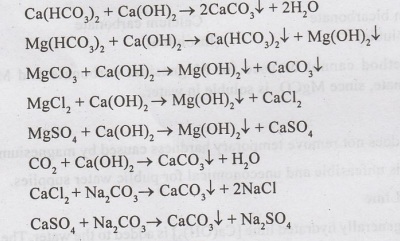
This
process removes the following:
•
Carbonate hardness (both Ca and Mg)
•
Non-carbonate hardness of Mg is converted into non carbonate hardness Ca, which
is removed by soda.
•
Removes free dissolved CO2
•
Equipments required for this process are :(Similar to coagulation process)
(i) Miving Tank - Lime and soda ash are added
to raw water and mixe
(ii) Flocculation
(iii)
Sedimentation - The precipitates formed are made to settle in the tank. The
detention time varies between 2 to 4 hours.
•
The dosage of lime and soda required for softening, depends upon the chemical
quality of water and the extent of hardness removal desired.
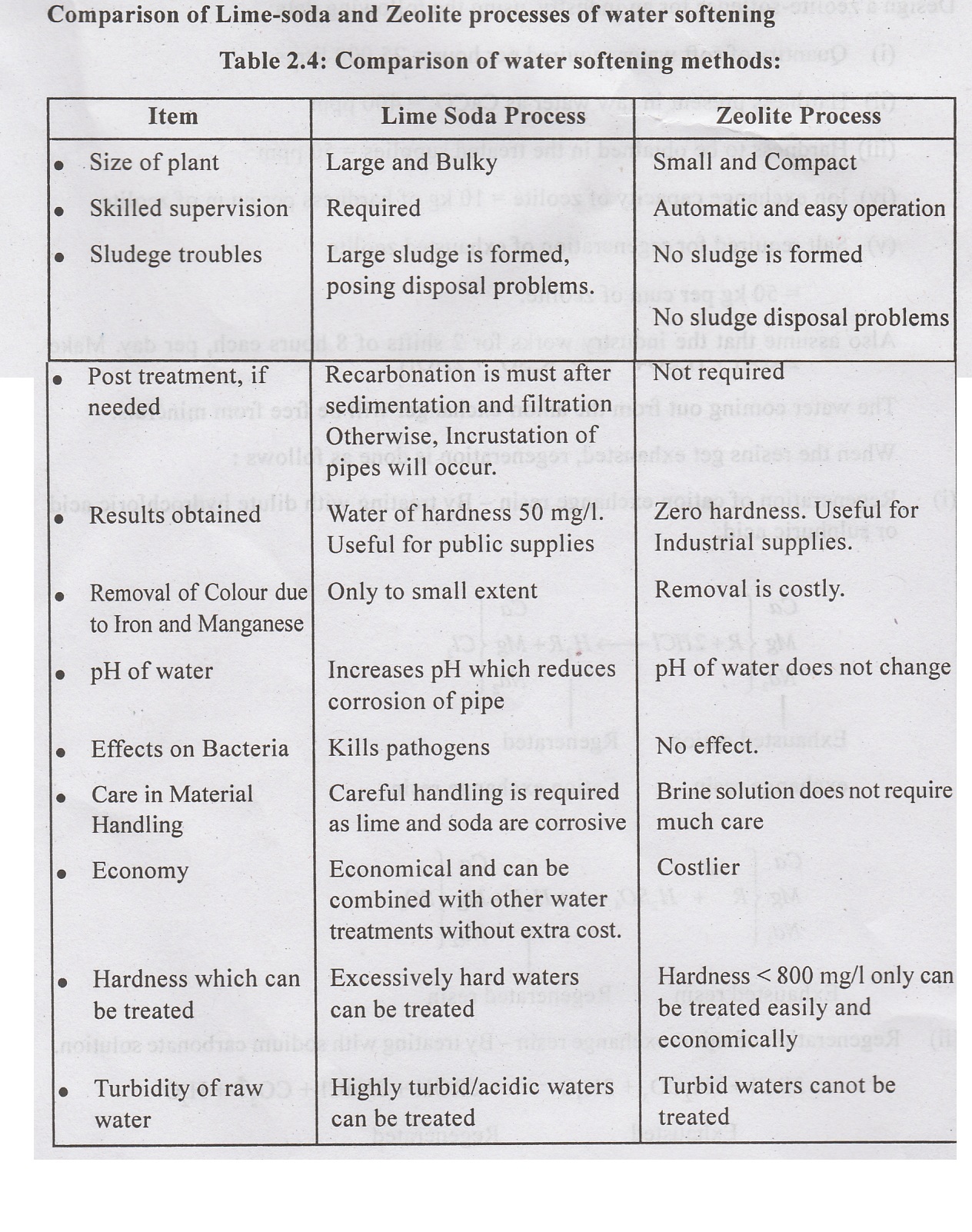
Advantages of Lime -soda process:
•
Economical
•
Easily combined with other water treatment methods.
•
Lime and soda used in combination with coagulants, reduces the dosage of
coagulants.
•
Increases pH of water and thus reduces corrosion of pipes.
•
Increases pH which kills the pathogens.
•
Reduces mineral content of water.
•
Removes iron and manganese to some extent.
Disadvantages of Lime-soda process:
•
Large quantity of sludge, precipitates of CaCO, and Mg(OH), is formed, which
requires proper disposal.
•
Careful operation and skilled supervision is required.
•
Recarbonation of water is required; otherwise it will cause incrustation of
pipe walls. (siiloss beau)
•
Zero hardness cannot be achieved. Hardness removal is only upto 50 mg/l.
Recarbonation of softened water:
The
very fine precipitates of calcium carbonate and magnesium hydroxide, may sometimes
not settle in the sedimentation tank. These particles may deposit on filters
and cause enlargement of sand grains i.e. incrustation of filter media and
distribution pipes.
To
prevent this, the softened water leaving the sedimentation tank should be
recarbonated by passing carbon dioxide gas.
In
Recarbonation process, the insoluable carbonates combine with carbon dioxide to
form soluable bicarbonates.
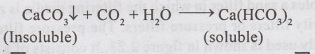
The
CO2 gas to be blown in water can be produced by burning coke, gas or
oil.
2. Zeolite or Base-exchange or
Cation-exchange Process:
•
Zeolite are naturai salts or clays, hydrated silicates of sodium and aluminium,
Issimodo or synthetic resins.
•
General formula is :
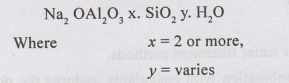
•
Zeolites have the property of exchanging their cations.
•
During water softening, Sodium ions of zeolite are exchanged with Calcium and
Magnesium ions in hard waters.
•
The chemical reactions involved are:
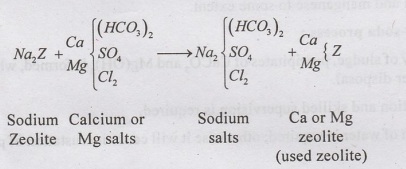
•
The Ca and Mg zeolite is regenerated by treating with 5 to 10% solution of
sodium chloride (Brine solution)
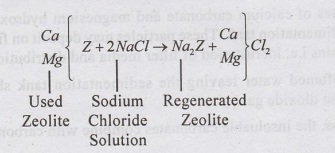
•
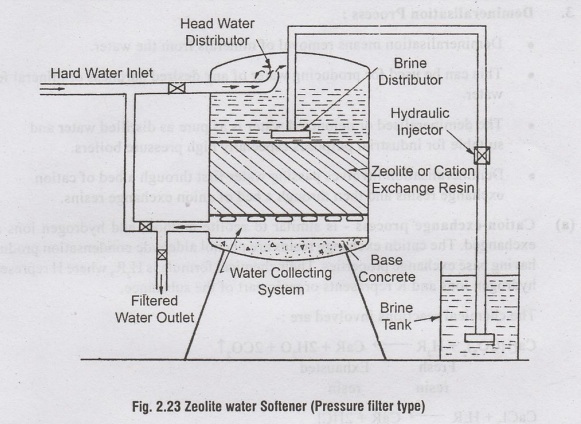
A zeolite softener resembles a sand filter in which the filtering media is
zeolite. They may be either gravity filters or pressure filters. The pressure
filter type zeolite softener commonly used is shown in figure 2.23. It consists
of a closed steel cylinder containing a bed of zeolite (0.75m to 2m thick). The
hard water 300 litres per sq.m per minute (0.1 to 0.3m/min) When the sodium
salts of zeolite are exhausted, it is regenerated by backwashing with 10% brine
solution with 10% bri (Nacl).
Advantages
(i)
Water of zero hardness can be achieved; useful for textile industries, boilers
(ii)
Plant is compact, automatic, easy to operate.
(iii)
No sludge is formed. No problem of sludge disposal.
(iv)
RMO (Running, Maintenance and operation) cost is less.
(v)
Also removes iron and manganese from water.
(vi)
No problem of incrustation of pipes.
Disadvantages:
(i)
Not suitable for treating turbid waters.
(ii)
Process leaves sodium bicarbonate in water, which causes foaming in industrial
or boiler feed waters.
(iii)
Costly and not suitable for treating water containing iron and manganese iron
or manganese zeolite cannot be regenerated back into sodium zeolit zeolite gets
wasted.
3. Demineralisation Process:
•
Demineralisation means removal of minerals from the water.
•
This can be used for producing water of any desired hardness or mineral
•
The demineralised or deionized water is as pure as distilled water and suitable
for industrial purposes especially high pressure boilers.
•
Demineralisation involves passing water first through a bed of cation exchange
resins and then through a bed of anion exchange resins.
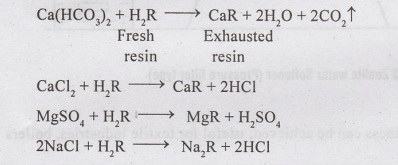
(a) Cation-exchange
process - is similar to zeolite method, and hydrogen ion
exchanged. The cation exchange resins are phenol aldehyde condensation pro
having base exchange properties. Their chemical formula is H,R, where H repre
hydrogen ions and R represents organic part of the substance.
The
chemical reactions involved are :-
In
the cation exchange process, acids [carbonic acid, hydrochloric acid, sulp acid
etc.] are formed, which are removed by the subsequent anion exchange pro
(b) Anion - exchange
Process:
The
anion-exchange resins are condensation products of amines with formalde with
anion exchange properties i.e., hydroxyl ions (OH) is exchanged. The cher
formula of resin is ROH, where OH represents hydroxyl ions and R represents org
part of the substance.
The
chemical reactions involved are:
ROH
+ HC1 →RCI + HOH
2ROH
+ H2SO4 → R2SO4+2HOH
The
water coming out from the anion-exchanger will be free from minerals. ziwiadio
When
the resins get exhausted, regeneration is done as follows:
(i)
Regeneration of cation exchange resin - By treating with dilute hydrochloric
acid or sulphuric acid.
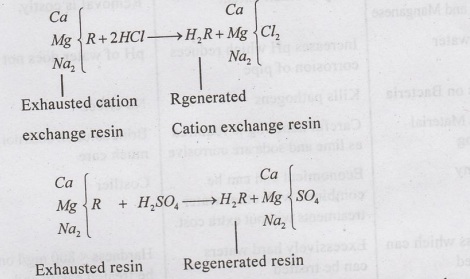
(ii)
Regeneration of anion-exchange resin - By treating with sodium carbonate
solution. 2RCI+ Na2CO3 + 2H2O → 2ROH + 2NaCl +
CO2↑ + H2O

Problem 2.6:
Design
a zeolite-softener for an industry, using the following data:
(i)
Quantity of soft water required per hour = 25,000 litres.
(ii)
Hardness present in raw water as CaCO, = 400 ppm
(iii)
Hardness to be obtained in the treated supplies = 50 ppm
(iv)
Ion exchange capacity of zeolite = 10 kg of hardness per cu.m of zeolite
(v)
Salt required for regeneration of exhausted zeolite
= 50 kg per cum of zeolite.
Also
assume that the industry works for 2 shifts of 8 hours each, per day. M suitable
assumption wherever needed.
Solution :
• Quantity of soft water required per shift of
8 hrs.
=
25,000 1/hr × 8 hr = 2,00,000 litres.
•
Hardness removal is upto 50 ppm out of total hardness of 400 ppm.
•
Percentage removal desired= 350/400 x100=87.5%
•
As the zeolite process reduces the hardness to zero; a part (87.5%) of raw
water is treated to obtain zero hardness and the balance (12.5%) is not treated
and added as raw water, to achieve hardness of 50 ppm.
The
quantity of water to be treated per shift
=
2 x 105 x 0.875 1.75 x 105 litres
The
amount of hardness to be removed per shift
=
[Quantity of water treated per shift in litres] x [hardness in mg/l]
=
(1.75 x 105) × 400 mg/l
=
70 x 106 mg = 70 kg.
The
quantity of zeolite resin required
Hardness
to be removed in Kg / Ion-exchange capacity of resin in Kg/cu.m
=
70 Kg / 10Kg/cu.m = 7 cu.m
Assume
number of units as 6 with one unit as standby.
Volumt
of one unit = 7 cu.m / 5 =1.4 cum
Provide
6 (5+1 standby) units with volume 1.4 cum, i.e. of area = 1m2 and depth =
Regeneration:
In
8 hours of shift time, assume regeneration process will take one hour and the
The
quantity of salt required for regeneration
=
50 kg/cum of zeolite
=
50 kg/cum x 7 cum
=
350 kg.
Using
10% brine solution (10 kg salt dissolved in water to make 100 kg solution
350
kg of salt will produce
350×100/10
= 3500 kg of water solution
=
3500 kg /1000kg/m3 =3.5 cum
Provide
two tanks of 1.75 cu.m capacity each. Assume the diameter of tank as m, then
the depth required
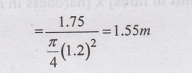
Using
free board = 0.15, Overall depth = 1.55 + 0.15 = 1.7m
Overall
tank size will be = 1.2 m dia x 1.7 m depth
Check for contact period
Flow
rate over zeolite bed
=
volume of water treated per shift /operation hours of zeolite
=
1.75×105 /7 litres/hr =25,000 litres/hr
Rate
of filtration = Flow rate of water over zeolite bed / surface area of zeolite
=
5,000-83.31/m2/min
=
0.083 m/minute > 0.3 m/min
It
is less than 0.3 m/min, Hence OK.
Contact
period (i.e. Average time of travel through the bed)
=
Depth / rate of filtration = 1.4 m/ 0.083 m/min
=
16.9 minutes > 7.5 minutes
Hence
design is OK
Water Supply And Wastewater Engineering: Unit II: Water Treatment : Tag: : Objectives, Methods, Working Principle, Advantages, Disadvantages - Water Softening
Related Topics
Related Subjects
Water Supply and Wastewater Engineering
CE3303 3rd Semester Civil Dept 2021 Regulation | Tag: 3rd Semester Civil Dept 2021 Regulation
