Water Supply And Wastewater Engineering: Unit I: Water Supply
Physical, Chemical and Bacteriological Analysis in water
Characteristics of Water
The coliform group of bacteria - Escherichia coli (E.Coli) is normally found in the intestinal tract of animals and human beings. E.Coli is not harmful, but its presence in water indicates the presence of other pathogenic micro organisms (like typhoid bacillus) in water
PHYSICAL,
CHEMICAL AND BACTERIOLOGICAL ANALYSIS
Bacteriological
impurities
The
presence of pathogens or disease causing microorganisms in water makes it
dangerous for human consumption.
The
coliform group of bacteria - Escherichia coli (E.Coli) is normally found in the
intestinal tract of animals and human beings. E.Coli is not harmful, but its
presence in water indicates the presence of other pathogenic micro organisms
(like typhoid bacillus) in water.
CHARACTERISTICS
OF WATER (WATER ANALYSIS)
1.
Physical Characteristics
2.
Chemical Characteristics nidolgomsem
3.
Microbiological Characteristics
1. Physical
Characteristics
(i)
Colour
(ii)
Taste and odour
(iii)
Temperature
(iv)
Turbidity
(i)
Colour
•
Colour in water is not harmful from health point of view but it may cause
staining/discolouration of clothes and is also objectionable from aesthetic
point of view.
•
Presence of dissolved organic matter, inorganic materials, algae, aquatic
microbes impart colour to water.
•
Colour is detected by naked eye or measured against hazen or platinic chloride
or cobalt scale (standard colour scale) using tintometer.
•
The tintometer has an eye piece with two holes, one for standard colour and
other for
•
For public water supplies, the colour of water on cobalt scale should be less
then 20.
Significance of colour
Colour
of water indicates the type of impurity present in water. Eg: of water
indicates the presence of manganese. (Isitatond) Inoigol
(ii) Taste and Odour
•
Taste and odour is due to the presence of mineral salts, industrial domestic
sewage, organic matter and microorganisms.
• It is difficult to measure taste and odour.
• It is measured in terms of Threshold Odour
Number which is the "volume of sample (in cm3) required to be added to 100
cc of odour free fresh water, where the mixture just starts giving smell in
diluted sample.
Example: If
6 cc of water is added to 100 cc fresh water - no odour is produced, while 7 cc
of water added to 100 cc fresh water gives odour. Then the threshold no. is 6.
• For public water supplies, the threshold no.
> 3
• Alternatively, an Osmoscope (i.e. inhaling
through 2 tubes) is used. Diluted water samples are prepared. One tube is
placed in diluted water sample. The 20 lbs other tube is placed in original
water sample. The mixture that gives the first detectable odour is taken as
threshold odour.
Odour
intensity (PO value)
-
The number of dilutions of fresh water is known as PO value or odour intensity.
PO value / Meaning
0
- No perceptible odour
1
- Very faint odour
2
- Faint odour, detectable
3
- Distinct odour, readily detectable
4
- Very distinct odour
5
- Strong / Intense odour
6
Extremely strong odour
(iii) Temperature
The
preferable temperature is 10° to 20°C
Temperature
higher than 25°C is objectionable.
Significance
Dissolution
of gases (dissolved oxygen) depends on temperature.
BOD
and biological (bacterial) activities in water are temperature dependant.
(iv)Turbidity (Optical property)
It
is the resistance of water to passage of light. It is caused due to suspended
and I colloidal solids in water.
The
permissible turbidity for domestic water - 5 to 10 ppm
Methods
of measuring turbidity of water:
(a)
Turbidity rod
(b)
Jackson's turbidimeter
(c)
Baylis turbidimeter
(d)
Nephelometers
(a)
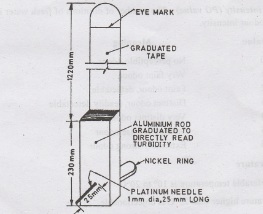
Turbidity rod
It
is a graduated aluminium rod attached to platinum needle and nickel ring at
bottom, graduated tape at top with position of eye marked.
The
Rod is inserted in turbid water. Reading is taken when the needle just
disappears. Turbidity is expressed in ppm.
(b)
Jackson's Turbidimeter
•
Used when turbidity > 100 ppm
•
It consists of a metal stand holding a metal container and graduated glass
tube.
•
A candle is placed below the standenado ai
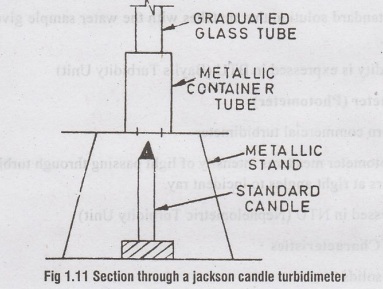
•
The image of flame is seen through turbid water in glass tube
•
The water level is increased till the flame just disappear
•
The height of water column is the Turbidity of water.
Expressed
in JTU (Jackson Turbidity Unit)
(c) Baylis
Turbidimeter
Very
accurate instrument used when Turbidity < 5 units
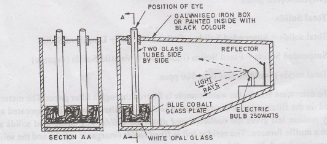
•
It consists of galvanised iron box with two glass tubes on one end and 250 watt
bulb with reflector on other end. The tubes are surrounded by blue cobalt
glass.
•
In one tube, standard solution and other tube, water sample are placed.
•
The standard solution is changed till it matches with the turbid water sample.
•
The standard solution that matches with the water sample gives the turbidity
value.
•
Turbidity is expressed in BTU (Baylis Turbidity Unit)
(d) Nephelometer
(Photometer)
•
Modern commercial turbidimeter
•
A photometer measures intensity of light passing through turbid water after it
scatters at right angles to incident ray.
•
Expressed in NTU (Nephelometric Turbidity Unit)
2. Chemical Characteristics
(i)
Total solids
(ii)
Chlorides
(iii)
Hardness
(iv)
pH value
(v)
Metals and other chemicals
(vi)
Nitrogen and its compounds
(vii)
Dissolved gases
(i) Total Solids
Total
solids = Suspended Solids + Colloidal Solids + Dissolved Solids
Suspended
Solids = Fixed Solids (Inorganic) + Volatile Solids (organic)
Total
solids should be less than 500 ppm
To
determine Solids, the water sample is filtered through a filter paper. The
material retained on the filter paper is the suspended solids. The filtered
water is evaporated and the day residue left gives the colloidal and dissolved
solids. The suspended solids are burnt in a muffle furnace. The residue left is
fixed solids (inorganic solids) and the solids that vaporizes is Volatile
solids (organic matter)
Significance
High
solids content indicates contamination and presence of excessive minerals.
Suspended
solids cause turbidity and imparts colour.
Dissolved
solids imparts colour, taste and makes it salty.
(ii) Chlorides
•
Presence of sodium chloride (Nacl) in water.
•
Source of chloride in water are from sewage effluents, mineral deposits and ingression
of salt water.
•
Chloride is determined by titration with standard silver nitrate using
potassium chromate as indicator.
•
The permissible level of chloride is 200 mg/l
Significance
Presence
of chloride in water indicates sewage contamination
(iii) Hardness
presence
of salts such as carbonates, bicarbonates, chlorides, sulphates of Calcium and
Magnesium.
Total
Hardness = Temporary (Carbonate) Harduess + Permanant (Non Carbonate)
Hardness
Temporary hardness (Carbonate hardness)
•
It is due to the presence of carbonates and bicarbonates of calcium and
Magnesium.
•
It can be easily removed by boiling or adding lime.
Permanent hardness (Non carbonate
hardness)
•
This is due to the presence of sulphates, chlorides and nitrates of Calcium and
Magnesium.
•
Special water softening methods are required to remove permanent hardness.
•
Hardness is expressed in degree

Methods
to determine Hardness:
(a) Clark's Method
Hardness
is found by determining the standard soap solution required to obtain permanent
lather with water sample of known volume at constant shaking.
(b) Hehner's Method
Temporary
Hardness - Determined by Titration with std soln.
H,SO, using methyl orange as indicator.
Permanent
Hardness - Standard Sodium Carbonate is added to
water sample and evaporated. The amount of Sodium Carbonate required in excess
to convert sulphate/ chloride into Carbonate gives the permanent hardness.
(c) Versenate Method
Hardness
is found by titration against Di-Ethylene diamine tetracitic acid (EDTA)
solution using Erichrome BlackT as indicator.
Excessive hardness is objectionable
because
•
Soap required is more
•
Scaling in boilers and heating systems from.gninoffo
•
Corrosion of pipes and plumbing fixtures
(iv) pH (Hydrogen ion
concentration)
Gives
the degree of acidity or alkalinity of water.
Water
molecule (H2O) dissociates into hydroxyl (OH-) and hydrogen (H+)
ions.
H2O→
(OH) + (H+)
(H+)
x (OH) = 10-14
For
pure water
H+=
OH = 10-7
pH
= 7 (neutral)
pH
= -log(H+)
pH
is the negative logarithm of H+ ions
If
pH < 7 - acidic
If
pH > 7 – alkaline
Significance of pH
•
Causes incrustation / tuberculation in pipes
•
Corrosiveness of water is pH dependent
•
Biological activities are pH dependent vd bovome
•
Water softening process depends on pH
•
Coagulation process depends on pH
Methods to determine pH
a) Colourimetric
Method
•
Indicator is added to water sample
•
Colour of water is compared to standard colours of known pH
•
Acidic range indicator - methyl red
•
Alkaline range indicator - phenolpthalein red
•
Standard colours are in the form of coloured glass disc and coloured charts
(b) Electrometric
method
•
A PH meter is used. The pH electrode is dipped in the water sample. pH value
can be read directly on the dial.
(v) Metals and other
chemical substances
(a)
Iron and Manganese
•
Iron causes hardness, bad taste, discoloration of clothes and plumbing
fixtures, heavy growth of crenothrix which leads to pipe clogging.
•
Iron can be found by simple calorimetric procedure using thiocyanate and
thioglycollic acid.
• Manganese imparts brownish / purplish
colour to water and stains plumbing fixtures
•
Manganese is found by matching the pink colour produced on oxidation to
permanganate. Sodium paraperiodate and ammonium persulphate are used to colour
both water sample and manganese colour standards.
(b)
Lead and Arsenic
•
Lead is a cumulative poison. Prolonged exposure can cause death.
•
Concentration of lead should be less than 0.05 mg/l
•
Lead is detected by 6 drops of sulphuric acid in water sample; white
precipitate formed indicates presence of lead.
• Arsenic causes Chronic
poisoning and is difficult to diagnose. Arsenic is removed by ion exchange
equipment using activated alumina. Determined by Heteropoly blue colorimetric
method by comparing stains on paper strips.
(c)
Fluorides and Iodides
•
Small concentrations of Fluoride and Iodides are useful to human
•
Iodine < 1 ppm - prevent goitre.
•
Fluoride < 1.2 ppm – prevent dental caries in children
•
Excess concentration > 3 ppm - Causes dental fluorosis or mottled enamel
• Fluorides
is estimated by colourimetric method ie., by developing a colour with zirconium
- alizarin reagent.
•
Iodide is determined by its ability to catalyze reduction of ceric ions by
arsenious acid.
1.29
(d) Barium and Boron
• Barium is toxic to heart, blood vessels and
nerves (Central Nervous System). It may cause 'borism'. Determined by
Colorimetric and Titrimetric methods.
•
Solution of Carmine acid in concentrated sulphuric acid changes colour from
bright red to bluish red or blue depending on the concentration of boron.
(e)
Cadmium and Hexavalent Chromium
• Cadmium is
toxic. It is discharged from electroplating plants. Cadmium forms red compound
with di-phenyl thiocarbzone and this is used for colourimetric determination of
cadmium.
• Chromium can cause cancer. It is discharged
from chromium plating units. It is determined colourimetrically by reaction
with diphenyl-carbazide in acid solution.
(f)
Sodium and Potassium
•
Human body maintains constant sodium content.
•
Excess quantities of sodium causes edema.
•
Sodium and potessium is determined by using Flame Photometer.
(vi) Nitrogen and its compounds
Forms
of nitrogen
(a)
Ammonical nitrogen (free ammonia)
(b)
Albuminoid nitrogen
(c)
Nitrite nitrogen
(d)
Nitrate
(a) Ammonical
Nitrogen
•
Free ammonia in water indiates presence of organic matter particularly human
and animal excreta, and discharge from gas industries.
•
Free ammonia is estimated by distillation method using Nessler's Reagent.
(b) Albuminoid
Nitrogen
•
It is presence indicates organic polluction in water
•
It is derived from aquatic plant and animal life.
•
Determined by adding potassium permangnate or sulphuric acid to water sample to
and boiling to liberate ammonia gas.
(e) Nitrite
•
It is an intermediate in the oxidation - reduction process of the Nitrogen
cycle.
•
Its presence indicates pllution.
•
Determined by treating with sulphanilic acid and matching the colour with
standard nitrite solution.
(d) Nitrate
•
This is the final stage of oxidation of Nitrogen compounds bise mi 560
•
The source of nitrate in water is from cultivation, manure and biological
activity in soil.
•
Excessive nitrate in water causes infant poisoning - Blue Baby syndrome -
methae moglobinemia.
•
It is determined by matching the colour produced with phenol-disulphonic acid.
(vii)
Dissolved Gases
(a) Dissolved Oxygen
(DO)
•
DO in water is from dissolution of oxygen from the atmosphere and
photosynthetic activities by algae and aquatic plants.
•
The oxygen dissolution in water is temperature dependant. DO will be less at
high temperature.
•
DO is required by aquatic plants and fishes.
•
Minimum DO that should be present in water is 4 ppm.
•
DO is an indicator of the unstable organic matter in water.
•
Excess DO causes corrosiveness of water.
•
However, if DO is less, it will affect the photosynthetic activity of aquatic
plants, and aquatic animals cannot survive without oxygen. The water will
become septic and dangerous.
(b) BOD - Biochemical
Oxygen Demand
•
It is a measure of the oxygen required by micro organisims to oxidize the
organic matter in water
•
BOD indicates the amount of unstabilised organic matter (pollution) in water.
•
In unpolluted waters, BOD will be less than 5 ppm
•
BOD test is conducted by incubating ng the water
(c) Carbon dioxide
•
CO2 in water is from atmosphere, decomposing organic waste and
underground sources.
•
It makes the water corrosive
•
This can be reduced by aeration of water
•
It is determined by using alkali with phenolphthalein indicator
(d) Hydrogen Sulphide
(H2S)
•
Mostly in ground waters; produced by the decomposition of organic matter and by
sulphate reducing bacteria.
•
It produces rotten egg smell
•
It is reduced by aeration
• H2S is determined by titration
with iodine.
3. Biological Characteristics
A
contaminated water is a host for micro organisims that may spread water-borne
diseases.
Various
micro organisms found in water are classified as
(a) Aquatic Plants
Water
weeds and algae are of great concern in water supply sources.
Excess
growth of algae and weeds causes the following problems:
(i)
They infect the lakes and prevent entry of sunlight and oxygen into water.
(ii)
They impart objectionable taste, odour and colour.
(iii)
Cause turbidity
(iv)
Clog the filter beds
(v)
Reduces the capacity of canals and pipes
(b) Aquatic animals
•
Fishes, amphibians, insects, spiders, snails, earthwarms, protozoans etc.
•
Some are resistant to chlorination
•
They cause intestinal infections, dysentry etc.
•
They also cause clogging of supply pipes.
(c) Fungi, Bacteria and Viruses
•
They modify pH of water and produce organic acids and ammonia
•
Decomposition or death of fungi imparts disagreeable taste and odour to water.
•
They are controlled by treatment of water with Chlorine or Copper Sulphate.
Viruses
•
They are infections agents and cause waterborne diseases
•
Bacteriophages viruses cause respiratory infections in children, Hepatitis,
Polio etc.
Bacteria

Different groups of bacteria that
are of importance are:
(i)
Coli form group / Coli-aerogenes (E.coli)
These
bacterias are harmless and are found in the intestines of animals and human
beings and are excreted with faeces. But, the presence of these bacteria
indicates the contamination of sewage in water and presence of pathogens.
Microbiological
investigations are done for E.Coli - Escherichia Coli which is used as the
indicator organisms. E.coli is found in the human intestine and is not harmful.
But the presence of E.coli in water confirms the presence of other disease
causing (pathogens) microorganisms in water.
Purpose of microbiological
examination of water
•
To detect the degree of pollution in water bodies
•
To assess the amount of treatment required to render the water safe.
•
To ascertain the efficiency of treatment at various stages.
•
To locate the cause of any sudden deterioration in water quality.
Coliform Index or E-coli Index
The
number of coliform bacterias present in the water sample is found by the
following procedure.
Fermentation
tubes are inoculated with varying portions of water sample. The reciprocal of
the smallest portion that shows positive result is the coliform Index.
For
example: Suppose a test shows the following results:

The Most Probable Number (MPN)
MPN
is defined as the bacterial density which if it had been actually present in
the sample under examination, would more frequently than any other, give the
observed analytical result.
MPN
is based on probabilities. Standard tables are available to determine MPN for
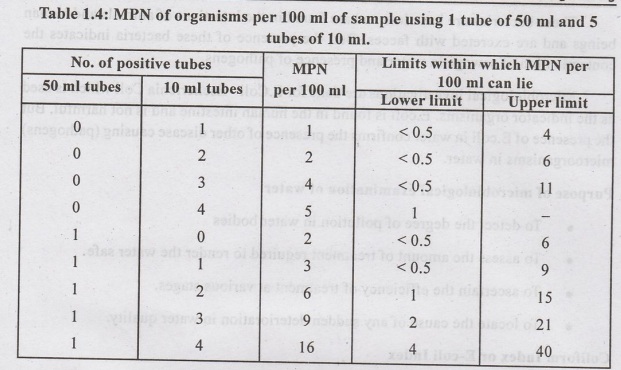
1. Total Count or Agar Plate Count
Test (Colony Count)
Test
is done to find the number of bacterial colonies growing on a medium incubated
for a specified time at a specified temperature.
The methods use are:
(i)
Colony count in nutrient agar after 3 day incubation at 20 to 22°C
(ii)
Colony count in nutrient agar after 24 hrs @ 37°C (common)
(iii)
Colony count after continuation of 37°C incubation for further 24 hours.
Water
sample after suitable dilution is taken in petridish. Molten nutrient agar is
added and incubated at 37°C in an incubator for 24 hrs.
Then,
bacterial colonies are counted using magnifying glass.
bevis
For Potable water; Total Count should not exceed 100 per ml.
2. The Coliform Test
Coliform
group includes all aerobic and facultative anaerobic bacteria
Test Techniques are:
(a)
Multiple tube fermentation technique
(b)
Membrane filter technique
(a) Multiple tube
fermantation technique: This consists of three stage tests:
(i)
Presumptive test
(ii)
Confirmed test
(iii)
Completed test
(i) Presumptive test
•
Fermentation (Durham) tubes are used
•
Measured volume of water sample is taken in two tubes.
•
Bite salt lactose broth is added and tubes are incubated at 37°C for 24 to 48
hrs.
•
Acid or gas formation after 24 to 48 hrs shows positive test.
•
This test alone is not reliable.
•
The tubes that gave positive results should be tested further to confirm the ad
presence of E.coli.
(ii) Confirmed Test
•
The positives tubes from presumptive test are further tested.
•
The presumptive culture is placed on MacConkey agar or Eosin-methylene blue
agar and incubated at 37°C for 24 hrs.
•
The growth of single discrete pure dark red bacterial Colonies results. positive
•
Alternatively, the culture can be transferred to another fermentation tube
containing brilliant green lactose broth and incubated at 37°C for 48 hrs.
•
Formation of gas after 48 hrs shows positive results. amotilos arb
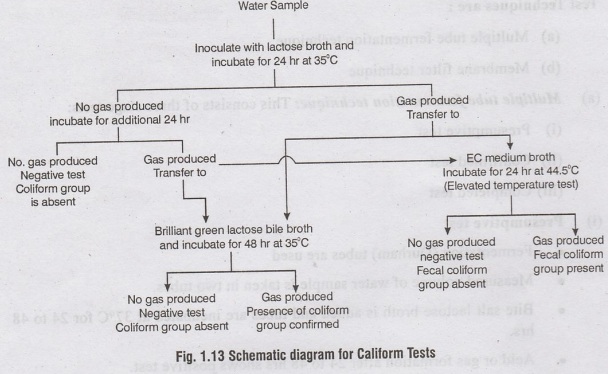
(iii) Completed test
•
Positive tubes from completed test are subject to further testing.
•
The Culture is placed on Endo or Eosin methylene blue plates and incubated at
37°C for 24 hrs.
•
Typical coliform colonies are transferred to Mac Conkey broth tube and nutrient
anelydis agar and incubated at 37°C for 48 hrs. Gas production shows positive
results
(b) Membrane filter technique
•
High precision technique
•
Filter membrane made of cellulose esters with microscopic pores are used.
•
The water sample is made to pass through the filter membrane which retains the
coliforms.
•
The membrane cultures are incubated at 37°C for 20 hours
•The
colonies exhibit a metallic luster and are counted under a microscope.

•
This test is not suitable for turbid water due to presence
Water Supply And Wastewater Engineering: Unit I: Water Supply : Tag: : Characteristics of Water - Physical, Chemical and Bacteriological Analysis in water
Related Topics
Related Subjects
Water Supply and Wastewater Engineering
CE3303 3rd Semester Civil Dept 2021 Regulation | Tag: 3rd Semester Civil Dept 2021 Regulation
