Water Supply And Wastewater Engineering: Unit II: Water Treatment
Disifection
Working Principle, Methods, Chlorination, Advantages | Water Treatment
The filtered water from the Slow or Rapid sand filters normally contains some harmful pathogenic (disease causing) bacteria. These bacteria must be killed in order to make the water safe for drinking.
DISINFECTION
The
filtered water from the Slow or Rapid sand filters normally contains some
harmful pathogenic (disease causing) bacteria. These bacteria must be killed in
order to make the water safe for drinking. The process of killing these harmful
bacteria is called disinfection and the chemicals used in this process are
called disinfectants. Disinfection not only kills the bacteria during treatment
but also prevents any recontamination during the distribution of water to the
consumer. The disinfectant chemicals used should therefore be able to give
residual sterilizing effect for a long period.
Minor
Methods of Disinfection
1. Boiling of water
(Sterilization): Most
effective method since boiling of water kills all the bacteria and
micro-organisms. However, this method is not feasible for large scale public
water supplies. During epidemics, the consumers are advised to boil the water
before drinking.
2. Treatment with
excess lime: Excess lime added increases the pH of
water to greater than 9.5, when E-coli present in water will die. The bacterial
removal efficiency is upto 99 to 100%. However, the excess lime added has to be
removed by re-carbonation or other suitable methods, before supplying it to the
consumers. The dosage of lime is between 10 to 20 ppm of Calcium Oxide.
3. Treatment with ozone: Ozone gas is faintly blue gas of pungent odour and is
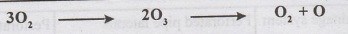
Because of its
instability, ozone readily breaks down into oxygen and nascent oxygen (O). The
nascent oxygen is a powerful oxidizing agent and removes the bacteria as well
as organic matter from water. The dosage is 2 to 3 ppm and contact period is 10
minutes. In addition, Ozone removes the colour, taste and odour from water.
However, it is very costly.
4. Treatment with
iodine and bromine: Addition of iodine and bromine to water
kills the pathogenic bacteria. The dosage is 8 ppm and contact period is 5
minutes. They are not used for treating large scale public supplies, but may be
used for small water supplies for army troops, private plants, swimming pools
Is etc.
4. Treatment with
ultra-violet rays: UV rays are effective in killing both
the active bacteria as well as spores. Though Sun is a powerful source of UV
rays, it requires large exposure area and long time. Hence UV rays are
generated by mercury vapour lamps enclosed in a quartz globe. Water should be
made to flow in thin films over the lamp and it should be colourless with
turbidity less than 15 ppm. This method is costly and hence not commonly used
except in private buildings, office buildings, institutions and swimming pools.
6. Treatment with
potassium permanganate:
Common
method in rural areas. Potassium permanganate (KMnO,) is dissolved in a bucket
of water and is mixed with the well water. The dosage is 1 to 2 mg/L with
contact period of 4 to 6 hours. Addition of potassium permanganate imparts pink
colour to water and the water should not be used during the first 48 hours.
7. Treatment with
silver, called Electro-Katadyn process:
Silver when immersed in water exerts an inhibiting action on bacterial life.
Silver ions, with or without activators (palladium or gold) are deposited on
particles of granular activated carbon. Bacteria laden water contacting the
silver impregnated carbon release minute quantities of silver - 25 to 40 ppb
which acts as a disinfectant. The contact time varies from 10 to 60 minutes.
Since silver is costly, this method is suitable for small installations or for
private individual houses.
Chlorination
Chlorine
is universally adopted for disinfecting public water supplies. It is cheap,
reliable, easy to handle, easily measurable and capable of providing residual
disinfecting effects for long periods, thus affording complete protection
against future recontamination of water in the distribution system. The only
disadvantage is that when used in excess, it When chlorine is added to water,
it forms hypochlorous acid or hypochlorite ions, which have immediate
disastrous effect on microorganisms.
Cl2+
H2O → HOCI (hypochlorous acid) + HCI
HOCI→
H+ (hydrogen ion) + OCH (Hypochlorite ions)
The
hypochlorous acid is unstable and may break into hydrogen ions and hypochlorite
ions. The above reaction is reversible and depends on pH of water. The sum of
hypochlorous acid, hypochlorite ions, and molecular chlorine existing in water
is called free available chlorine. Out of these, hypochlorous acid (HOCI) is
more destructive and the pH of water is maintained slightly less than 7, so as
to control the dissociation of HOCI.
Moreover,
the chlorine will immediately react with ammonia present in water to form
chloramines,

The
chloramines formed are stable and are found to possess disinfecting properties.
When the added chlorine has consumed all the ammonia available in water, then
it will persist as free chlorine. The combination of chlorine with ammonia in
the form of chloramines is called the Combined Chlorine, and is less effective
in causing disinfection compared to free chlorine.
The
free chlorine as well as the combined chlorine (chloramines) will cause
germicidal action on bacteria and pathogens. The free chlorine will
instantaneously kill the pathogens, while the combined chlorine will provide
long term germicidal effect.
In
general, most of the waters are satisfactorily disinfected if the free chlorine
residual is about 0.2 mg/l, 10 minutes after the chlorine is applied.
Various
forms in which chlorine can be applied:
•
Liquid chlorine or chlorine gas
•
Hypochlorites or bleaching powder
• Chlorine tablets
•
Chloramines ie., mixture of ammonia and chlorine
•
Chlorine dioxide
(i) Bleaching powder:
Bleaching powder or calcium hypochlorite Ca(OCI), is a chlorinated lime
containing about 33% of available chlorine. It loses its strength during
storage or exposure of air. It is therefore used only on small installations or
under emergency conditions.
The
process of chlorination with hypochlorites is known as hypochlorination. ovito
Commerical compounds such as HTH (high test hypochlorites), Pittclor, Pittcide,
no Hoodchlor etc are used instead of bleaching powder. The HTH has chlorine
content of 65 to 70%. Hypochlorites are applied to water as a solution by means
of hypochlorite feeding apparatus. It consists of a solution tank connected to
a constant level feeding tank. The dosage of the solution is adjusted by means
of an adjustable pinch clamp.
(ii) Chloramines: Chloramines, the combination of ammonia
and chlorine are widely used as they produce a more stable disinfecting
residual than produced by chlorine alone. In this treatment, ammonia is added
to water just before the chlorine is applied. The usual proportions are 1 part
of ammonia to 4.5 parts of chlorine by weight. Ammonia may be used in the form
of gas or liquid or ammonium sulphate or ammonium chloride. Since the
disinfecting action of chloramines is slower than chlorine alone, a contact
period of 2 hours should be provided before the water is used.
(iii) Free Chlorine:
Chlorine is generally applied in gaseous form or in liquid form. Gaseous
chlorine is a greenish-yellow poisonous substance, with a typical odour and is
2.48 times heavier than air. Liquid chlorine is amber coloured oily liquid and
about 1.44 times heavier than water. Unconfined liquid chlorine rapidly onino
vapourises to gas, 1 volume of liquid yields 462 volumes of gas. Chlorine is
stored and supplied in liquid form in metal containers under pressure. Since
liquid chlorine is highly corrosive, the cylinders containing liquid chlorine
are provided with special fittings. Chlorine gas is a respiratory irritant and
it can cause varying degrees of irritation to skin, mucous membranes and
respiratory system. Chlorine cylinders should be stored in a cool
well-ventilated room. The chlorine dose depends upon : Organic matter present
in water, pH of water, amount of carbon dioxide present in water, temperature
and time of contact.
(iv) Chlorine
Dioxide: Bactericidal properties of chlorine dioxide is
greater than chlorine. The chlorine dioxide gas is unstable, and is therefore
produced at the

It
is harmless in aqueous solution. It does not react with organic materials to
produce any harmful substances. It has greater oxidizing ability than chlorine.
The dosage varies from 0.5 to 1.5 ppm and is not affected by variations in pH.
However due to its high cost of production, it is rarely used.
Forms of Chlorination
Depending
upon the stage at which chlorine is applied to water, chlorination may be of
the following forms:
(i) Plain chlorination:
The application of chlorine to raw or untreated water supply as it enters the
distribution system. It also includes the chlorination of raw waters in tanks
or reservoirs to check the growth of weeds, organic matter, algae and bacteria.
It also removes colour and odour from water. This is done when water is
relatively clear with turbidities less than 20 to 30 ppm. The normal dose is
between 0.5 to 1 ppm.
(ii)
Pre-chlorination: It is the application of chlorine to
water before its treatment yllups (filtration or sedimentation). This helps in
reducing the amount of coagulants required because of the oxidation of organic
matter by chlorine. The dosage of chlorine is adjusted such that the chlorine
residual is 0.1 to 0.5 ppm.
Advantages of pre-chlorination:
•
It reduces the quantity of coagulants required
•
It reduces the bacterial load on filters
•
It controls algae and plankton growth in basins and filters
•
It eliminates tastes and odours
(iii)
Post-chlorination: It is the application of chlorine in
water after its treatment. This is the standard procedure followed in which
chlorine is added to water as it leaves the rapid sand filters and before it
enters the distribution system. The dose of the chlorine should be so adjusted
that the residual chlorine is about 0.1 to 0.2 ppm. It is useful for protection
against recontamination during distribution.
(iv)
Double or multiple chlorination: It refers to the
application of chlorine at two or more points in the purification process.
Generally, double chlorination is resorted to, in which chlorine is applied
just before water enters the sedimentation tanks, and after it leaves the
filter plants. This is done specially when raw water is highly The advantages
of double chlorination are similar to those of pre-chlorination with greater
factor of safety against pathogenic microorganisms.
(v) Break Point
Chlorination:
When chlorine is applied to water, two actions take place one after the other:
•
Chlorine acts as disinfectant and kills bacteria.
•
Chlorine acts as oxidizing agent and it oxidizes the organic matter.
Chlorine Demand:
Chlorine compounds are good oxidizing agents and hence they react with organic
and inorganic impurities present in water, before the disinfection is achieved.
The amount of chlorine consumed in the oxidation of these impurities is known
as chlorine demand of water. Only after the chlorine demand of water is
fulfilled, the chlorine will appear as free available chlorine to kill the
pathogenic microorganisms. Chlorine demand is therefore, the difference between
the amount of chlorine added to water and the quantity of free available
chlorine remaining at the end of the specified contact period.
Break point chlorination
is a term which gives us an idea of the extent of chlorine to be added to the
water. In fact, it represents, that much
dose of chlorination, beyond which any further addition of chlorine will
equally appear as free residual chlorine.
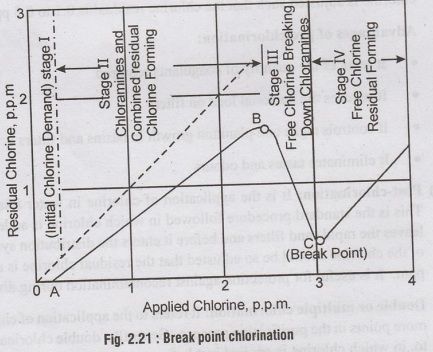
When
chlorine is added to pure water which has no organic impurities But, water
generally has some impurities or chlorine demand and as such results in curve
ABC, shown in figure 2.21. When chlorine is added to the water, it first reacts
with the ammonia present in the water, so as to form chloramines. The residual
chlorine curve (AB) therefore increases with the applied chlorine dosage but
shall be slightly less than the applied chlorine as some chlorine is consumed
for killing the bacteria. If the addition of chlorine is continued abizorby
beyond the point B, chlorine oxidizes the organic impurities in water and
therefore, the residual chlorine curve falls down, (BC). This point
"C" is called the break point, as any chlorine that is added to water
beyond this point, breaks through the water and totally appears as residual
chlorine. The addition of chlorine beyond break point is called break point
chlorination.
General
practice is to add chlorine beyond the break point and ensure a residual of 0.2
to 0.3 mg/l of free chlorine.
(vi) Super chlorination: Super-chlorination
is the application of excessive amount of chlorine (5 to 15 mg/l) to the water
to give about 1 to 2 mg/l of residue beyond the break point. This may be
required in some special cases of highly polluted waters containing high
concentration of organic impurities, or during pinsgro di epidemics of water
borne diseases or when water contains cysts of E. histolytica (organism causing
amoebic dysentery). Sometimes, even higher doses may be used and the resultant
water is dechlorinated before distribution.
Water Supply And Wastewater Engineering: Unit II: Water Treatment : Tag: : Working Principle, Methods, Chlorination, Advantages | Water Treatment - Disifection
Related Topics
Related Subjects
Water Supply and Wastewater Engineering
CE3303 3rd Semester Civil Dept 2021 Regulation | Tag: 3rd Semester Civil Dept 2021 Regulation
