Water Supply And Wastewater Engineering: Unit II: Water Treatment
Desalination Process
Methods, Working Principle, Advantages | Water Treatment
Due to the scarcity of fresh water, it has become necessary to convert salt water into potable fresh water, and the process is called Desalination. It also reduces the Total Dissolved Solids (TDS) in water.
DESALINATION
PROCESS
•
Only 0.5% of the earth's water is potable. The remaining 97% is ocean water and
2.5% is brakish water.
•
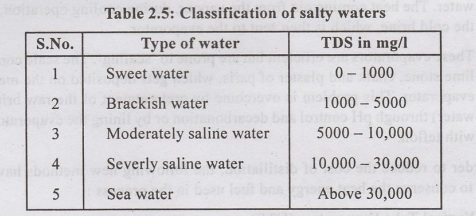
Natural water can be classified according to their TDS values.
Due
to the scarcity of fresh water, it has become necessary to convert salt water
into potable fresh water, and the process is called Desalination. It also
reduces the Total Dissolved Solids (TDS) in water.
Desalination
is a costly process. The water obtained by desalination proves much costlier
than the naturally available - treated water. Research is going on to reduce
the cost of desalination.
Methods of Desalination:
The
various methods which are generally adopted for the conversion of salt water
into fresh water are enumerated below:
(1) Desalination by evaporation and
distillation.
(2)
Electrodialysis method
(3)
Reverse Osmosis method
(4)
Freezing process
(5)
Solar distillation method and
(6)
Other methods.
1. Desalination by evaporation and
distillation:
This
is the most commonly used method of desalination. In this method, Sea or saline
water is boiled in giant vessels called evaporators, to produce water vapours
which are caught and condensed into fresh water.
•
An evaporator consists of a metal box in which the salt water (brine) is heated
by a nest of pipes carrying very hot steam.
•
Heat passes from the steam through the pipe walls and boils the brine.
•
The boiling brine evaporates and the vapour is led into a second box, where
another nest of pipes, filled with cold brine, condenses it to fresh and pure
water. The heat coming out from the vapour, during cooling operation, warms the
cold brine, which is then sent to the evaporator.
•
These evaporators are efficient but are prone to 'scaling'. The scale consists
of lime stone, chalk and plaster of paris, which gets deposited on the metal
box evaporator. This problem is overcome by pretreatment of the raw brine (salt
water) through pH control and decarbonation or by lining the evaporator tubes
with teflon.
In
order to reduce the cost of distillation, the following new methods have been
developed to conserve the heat energy and fuel used in the process:
(i)
Vertical Tube Evaporators (VTE)
(ii)
Multistage Flash Evaporators (MSF)
(iii)
Multieffect Multistage flash evaporators (MEMS)
(iv)
Combined process (VTE / MSF)
Vertical Tube
Evaporators (VTE)
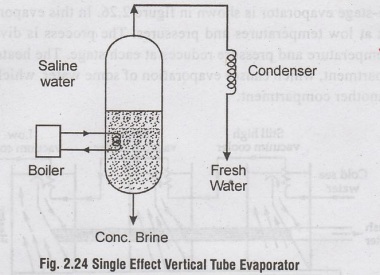
•
A single effect evaporator is shown in Figure.2.24 oil to aboston
•
The The steam from boiler heats and vaporises the salt water.
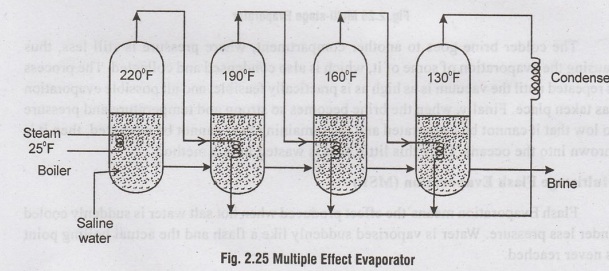
A
Multiple Effect Evaporator is shown in Figure 2.25. In this, the water
evaporates at the highest pressure in the first effect. The vapour is condensed
in the second effect to evaporate an equal amount of vapour. Similarly, the
third evaporator acts as condenser for the second and so on. The temperature
and pressures in the successive evaporators will go on reducing so that salt
water may boil at low temperature and pressure.
Multistage
Evaporation
Water
can be boiled at low temperatures, by reducing the pressure. The hot brine can
be vaporised to a certain extent at a certain pressure; the colder brine left
can also be vaporised at still lower pressures and so on. This principle is
used in 'multi-stage' evaporators i.e. water is evaporated - condensed again
and again in various stages. This technique efficiently utilises the heat
energy.
A
multi-stage evaporator is shown in figure 2.26. In this evaporator, the
operation is carried out at low temperatures and pressures. The process is
divided into different stages. The temperature and pressure reduces at each
stage. The heated brine is led into the first compartment, which causes
evaporation of some water, which is condensed and collected in another
compartment.
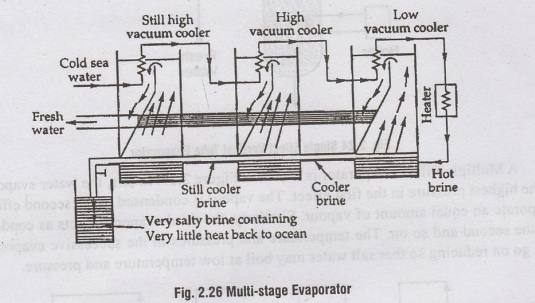
The
colder brine goes to another compartment, where pressure is still less, thus
causing the evaporation of some of it, which is also condensed and collected.
The process is repeated until the vacuum is as high as is practically feasible,
and all possible evaporation has taken place. Finally, when the brine becomes
so strong and temperature and pressure so low that it cannot be evaporated and
the remaining heat cannot be extracted, then it is thrown into the oceans. Only
this little heat is wasted in this method.
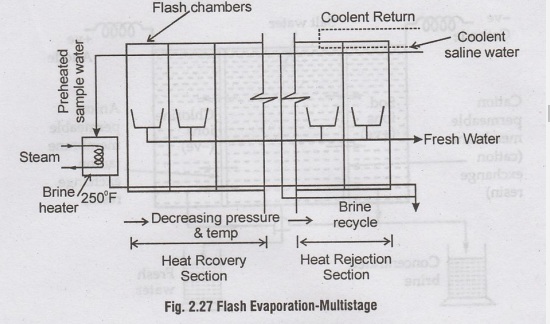
Multistage Flash
Evaporation (MSF)
Flash
Evaporation means the effect produced when hot salt water is suddenly cooled
under less pressure. Water is vaporised suddenly like a flash and the actual
boiling point is never reached.
The
salt water is heated to the highest temperature using a heater. The hot brine
under pressure is sprayed through nozzle into the first chamber which is at a
lower pressure and cooler. The sudden change produces the flash action and the
water will evaporate immediately. The salt water passes through a number of
compartments (flash chambers) wherein flashing of the brine occurs at
successively low pressures. The vapour released in flashing each stage
condenses to heat the incoming sea water and gives fresh water
Multieffect
Multistage Flash Evaporation (MEMS)
In
this method, the overall temperature flashing range is broken into a number of
temperature intervals. Within each interval, an individual single-effect
multistage unit with its own heat input, heat recovery, heat rejection and
recycling stream operates. The first effect receives heat from the external
source while for the subsequent effects, the heat from the previous effect is
rejected to the brine of the next effect. This utilises the heat input
efficiently.
2. Desalination by Electrodialysis
method:
In
salt water, H,O molecules are bonded together with sodium and chlorine ions.
These hydrogen-bonds between the H2O molecules and Na+ and Cl- ions must be
broken up, in order to separate the salt from water. These bonds where broken
by heat in the "method of distillation"; while in the
"electrodialysis method", these bonds are broken with the help of
electricity.
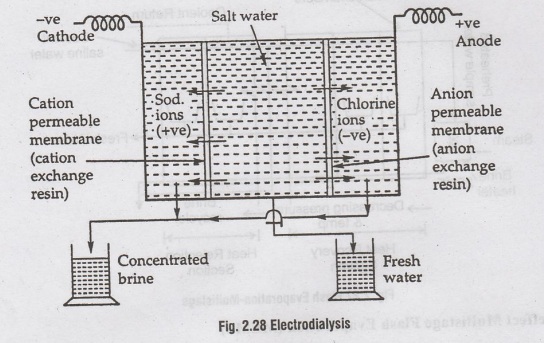
When
an electric-current is passed through the salt-solution, the sodium and
chlorine ions get freed from water molecules, and they start moving towards
their oppositely charged electric poles. In other words, the +vely charged
sodium ions will move towards the - ve pole.i.e., cathode, and the - vely
charged chlorine ions will move towards the +ve anode. If these cations (+ ve
ions) and anions (- ve ions) are allowed to segregate in different
compartments, what is left, is fresh-water.
The
segregation is achieved by means of thin plastic like sheets called
'membranes'.
A
cell consists of a pair of cation and anion membranes
installed in parallel between a pair of electrodes.
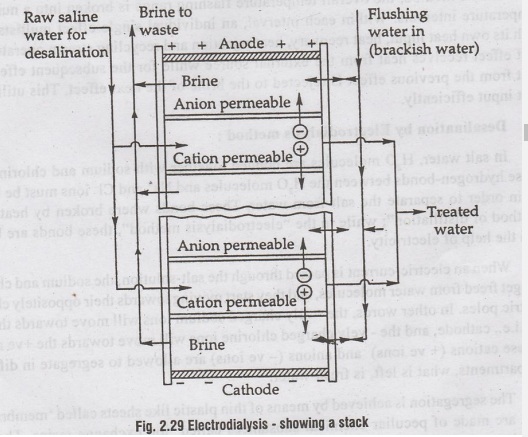
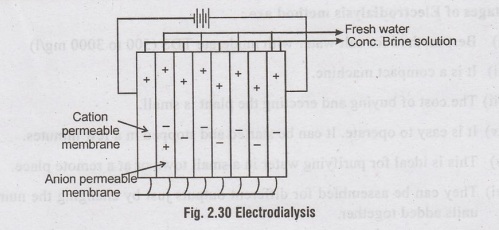
A
stack consists of a number of membrane pairs installed
parallel between a pair of electrodes (with spacers between each membrane).
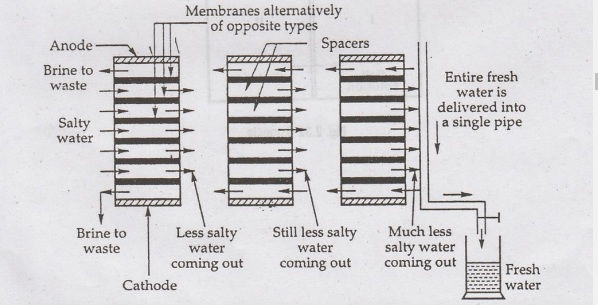
For
a big plant, stacks are hydraulically connected together, usually in parallel
(sometimes in series, also), as shown in figure 2.31. In such a plant,
therefore, a large number of small separations are hooked up together in
parallel; and alternate kinds of membrane are stacked with thin spacers in
between each pair of opposites. Pores give excess at each level, so that salt
water could be forced in between the membranes, while less salty water could be
pumped out at the other side, which can be recirculated through other stacks of
membranes. Since one stack of membranes usually removes 50% salinity, very fine
water could be produced in this manner, using three to four stacks.
Advantages of Electrodialysis
method are:
(i) Best method for salt water with moderate
TDS (500 to 3000 mg/l)
(ii) It is a compact machine.
(iii)
The cost of buying and erecting the plant is small.
(iv)
It is easy to operate. It can be started and stopped in a few minutes.
(v)
This is ideal for purifying water in a small town or at a remote place.
(vi)
They can be assembled for different outputs just by changing the number of
units added together.
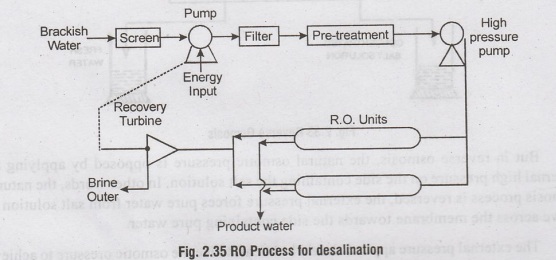
3. Desalination by Reverse Osmosis
process :
In
this method of desalination, the water molecules and the salt ions are
separated by forcing the salt solution against a 'semi-permeable membrane'
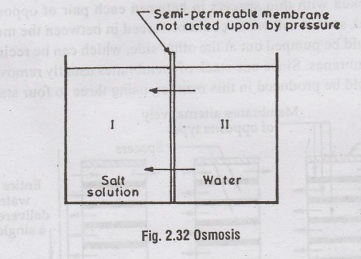
barrier, which permits the flow of water through itself but stops the salt. In
Osmosis, when salt solution is separated from pure water by a semipermeable
membrane, the pure water flows across the membrane until the pressure on the
pure water side become equal to the osmotic pressure of the salt solution.
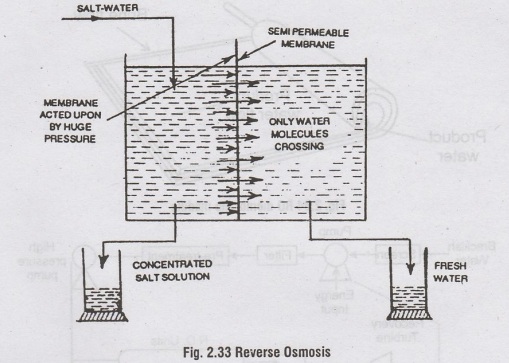
But
in reverse osmosis, the natural osmotic pressure is opposed by applying an
external high pressure on the side containing the salt solution. In other
words, the natural osmosis process is reversed, the external pressure forces
pure water from salt solution to move across the membrane towards the side
containing pure water.
The
external pressure applied should be atleast twice the osmotic pressure to
achieve
a
feasible flow. RO is usually operated at about 1000 Kg/cm2
The
semi-permeable membranes are thin but dense and strong enough to withstand the
high external pressure.
The
classification of membranes based on pore size and particle size removed, is
given below:
(i)
Micro filtration (MF)
(ii)
Ultra filtration (UF)
(iii)
Nano filtration (NF); and
(iv)
Reverse Osmosis (RO)
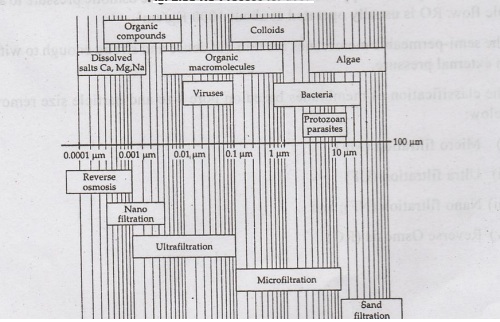
•
Microfiltration (MF) and Ultra Filtration (UF) are microporous membranes which
remove suspended small size particles by physical separation. They are low
pressure processes.
•
Nano filtration (NF) and Reverse Osmosis (RO) remove dissolved salts ions by
osmosis. NF is known as low-pressure reverse osmosis. NF and RO are high
pressure processes and the separation is by diffusion. RO rejects particles of
size 0.0001 μm, whereas NF rejects particles of size greater than 0.001 μm
(nanometer)
Reverse
Osmosis can be used for desalination with low pressure membranes (as Nano
filtration) of moderately salty waters (TDS of 1000 to 10000 mg/l); and with
high pressure membranes for severely saline waters containing TDS above 10000
mg/l. Sufficiently good quality water containing TDS within 500 mg/l can be
obtained by this method.
4. Desalination by Freezing
process: This method is based upon the principle that when
salt water freezes, the ice formed in the beginning is almost free from salt.
This ice, when melted, can give us good water. The quality of water obtained is
20 satisfactory, but the cost of production is high and prohibitive. A freezing
process involves cooling of the incoming sea water, freezing it to ice,
separating the ice and brine liquor, melting the ice to give fresh water and
using the concentrated brine to chill the incoming sea water. Liquefied
hydrocarbons (n-butane) can be used as refrigerants. In direct contact
refrigerating system, the refrigerated butane is mixed with the salt water.
Alternatively, pressure freezing; a high-boiling hydrocarbon is frozen and
mixed with prechilled sea water.
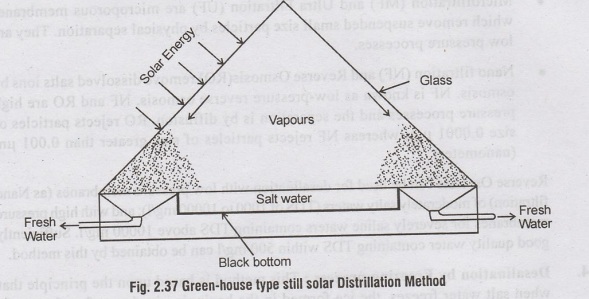
5. Desalination by Solar
Evaporation Method :
The
other desalination methods use thermal energy or mechanical power. But solar
evaporation uses solar radiation as the source of heat energy. The equipment
required is simple. The figure 2.37 shows a green-house type still, which may
yield about 5 kg of fresh water/day/m2 of basin surface, under good sunshine
conditions. Salt water is contained in a blacked bottom shallow through which
absorbs the solar energy. The vapours rise and condence on the glass or plastic
surface, so inclined as to cause the collected water to flow to a common
reservoir. The glass surface must be frequently cleaned off dust etc. to keep
them transparent.
6. Other methods of desalination:
In this method, propane gas is allowed to combine with salt water under
controlled conditions of temperature and pressure. A chemical reaction takes
place between salt water and propane gas at temperatures higher than the
freezing point of water, forming ice like crystals. These crystals reject the
salt and accept only pure water in their composition. These crystals are
separated from the brine, washed and decomposed to form water. The propane gas
released
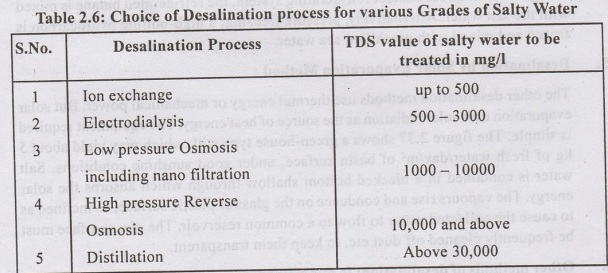
Selection of a particular method of
Desalination :
2200
Selection of the correct process, especially in the 500 - 5000 mg/l range
(TDS), requires careful evaluation of process efficiency, plant capital and
running cost. The application of the various desalination processes has been in
the following categories of TDS waters.
Water Supply And Wastewater Engineering: Unit II: Water Treatment : Tag: : Methods, Working Principle, Advantages | Water Treatment - Desalination Process
Related Topics
Related Subjects
Water Supply and Wastewater Engineering
CE3303 3rd Semester Civil Dept 2021 Regulation | Tag: 3rd Semester Civil Dept 2021 Regulation
