Water Supply And Wastewater Engineering: Unit IV: Planning And Design Of Sewerage System
Corrosion in Sewers & Concrete Sewer Pipe
Sewerage System
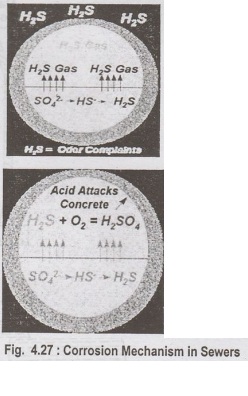
Bacteria in the slime under flowing sewage convert sulphates in the sewage into sulphides. Sulphides in the liquid make their way to the surface of the sewage and released into the sewer atmosphere as hydrogen sulphide (H,S) gas.
CORROSION
IN SEWERS
Bacteria
in the slime under flowing sewage convert sulphates in the sewage into
sulphides. Sulphides in the liquid make their way to the surface of the sewage
and released into the sewer atmosphere as hydrogen sulphide (H,S) gas.
H2S
gas in atmosphere makes contact with slime in the crown of the sewer, which
contains more bacteria. Bacterial action converts, H,S gas to sulphuric acid
which causes corrosion in the crown of the pipe and this corrosion is also
called crown corrosion. If pipe material is of corrodible nature sulphuric acid
attacks the pipe material and causing ultimate failure.
UNDERSTANDING
CONCRETE SEWER PIPE CORROSION
Brief History
Concrete
sewer pipe corrosion was first observed towards the end of the 19th century
when it was rightly concluded that sulphuric acid was the corrosive agent
however it wasn't until the 1940's that the biological nature of the concrete
corrosion process was established through work undertaken in Australia and the
USA when the bacterium "Thiobacillus concretivorous", (later renamed
Acidithiobacillus Thiooxidans) was identified amongst the acidic corrosion
products. Today we know that there are many bacterial and fungal species
involved in the concrete sewer pipe corrosion cycle.
Microbial
induced corrosion (MIC) of concrete sewers was not regarded as a significant
issue until the 1980's when corrosion rates in sewers increased significantly
in the USA, Europe and Australia. At this time tighter controls placed on
industrial wastewaters to be discharged to the sewer system (e.g. the US Clean
Water Act) led to significantly lower levels of biologically toxic metals such
as lead, mercury and arsenic in the sewer system and as a consequence bacterial
levels (and consequently MIC) increased dramatically.
Modern
day increases in corrosion activity are also linked to:
• increased sewage temperature, (due to
increasing use of hot water in domestic situations)
• increased use of sulphate containing
detergents
• increase in sewer line lengths (and
hence sewage residence times) om sd reinforced concrete sewer pipe is currently
considered one of the most serious egunotand costly problems currently
affecting the world's sewer infrastructure with the global repair bill for MIC
corrosion of sewer piping estimated to be in the order of billions of dollars
per year. on pri prihub noizol
jonsini od bollment
nolls
Concrete sewer pipe is corroded by acids produced in the sewer from chemical
and microbial processes. Microbial induced corrosion (MIC) however is
responsible for most of the corrosion that takes place.
The
basic sewer processes that drive concrete corrosion activity are illustrated in
Fig. 4.28. The corrosion cycle begins in the wastewater stream. Colonies of
anaerobic sulphate reducing bacteria (SRB) active in biofilm layers that line
the submerged sewer walls reduce sulphates and oxidise biodegradable organic
carbon producing hydrogen sulphide and carbon dioxide:
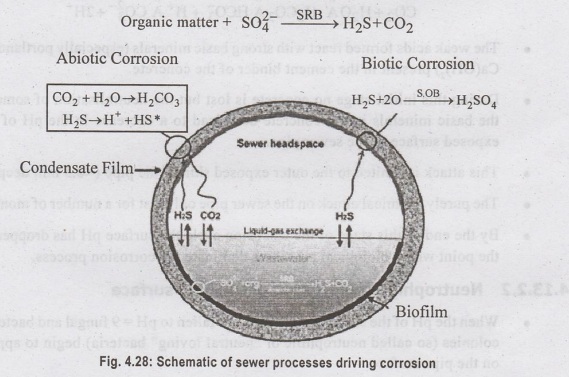
HS
and CO2 are transported through the biofilm into the wastewater
stream where some is volatilized into the sewer headspace. The gases diffuse
through the headspace atmosphere and dissolve into moisture present in the
concrete pores of the upper section of the sewer pipe. The dissolved H2S and
organic material deposited on the sewer pipe surface by washing or condensing
aerosols are the nutrients which drive the microbial corrosion process.
Corrosion
of a concrete sewer pipe proceeds over a number of stages from the time of
installation until pipe failure. As a result the rate at which the pipe is
corroded changes with time. The following discussion is a brief summary of that
process.
1 Chemical corrosion during the
initial months
•
When a concrete sewer pipe is first manufactured and installed the interior bas
loin surface of the pipe is too alkaline (pH>10.5) for bacterial or fungal
colonisation admol to take place.
•
Under these circumstances only the chemical corrosion of the pipe is possible.
•
H2S and CO2 dissolve in the concrete pore water to form
weak acids:
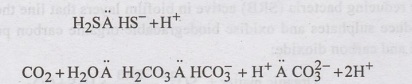
•
The weak acids formed react with strong basic minerals (especially portlandite,
Ca(OH),) present in the cement binder of the concrete.
•
During this initial stage no concrete is lost but the neutralisation of some of
the basic minerals in the concrete does lead to a decrease in the pH of the
exposed surface of the sewer pipe.
•
This attack is limited to the outer exposed skin of the pipe (<0.5 mm deep)
•
The purely chemical attack on the sewer pipe only last for a number of months.
•
By the end of this stage of the corrosion cycle the surface pH has dropped to
the point where biological processes dominate the corrosion process.
2 Neutrophilic colonisation of the
pipe surface
•
When the pH of the surface of the pipe has fallen to pH = 9 fungal and
bacterial colonies (so called neutrophilic or "neutral loving"
bacteria) begin to appear on the pipe surface.
•
As the surface of the pipe becomes more acidic successive waves of bacteria
more suited to lower pH conditions will dominate the pipe surface.
•
Neutrophilic bacteria produce carboxylic acids as well as sulphuric acid from
the oxidation of a number of sulphur species present on the pipe surface.
•
The production of these acids acts to further lower the pH of the sewer pipe
surface but no measurable loss of mass has as yet occurred.
3 Sound concrete loss
begins.
•
When the pH of the surface of the sewer pipe falls to pH 6 significant
conversion of sound concrete to corroded product (mostly gypsum) begins.
•
The time taken to reach this point in the corrosion cycle varies from site to
site (depending on the environmental conditions present) but will generally be
between 6 months to 2 years from the date of installation.
•
Once mass loss begins the rate at which concrete is lost per year remains
constant for the remainder of the pipe's service (if sewer conditions remain
constant).
•
The rate at which concrete is lost however varies from site to site and is
dependent on a number of environmental factors.
•
The surface pH will continue to fall after mass loss begins but the rate at
which it falls slows.
•
As sound concrete loss continues a layer of corroded product builds on the pipe
surface however this layer does not affect level of corrosion activity and the
rate of corrosion remains constant.
•
When the amount of concrete lost over time is plotted the corrosion function
takes the following general form:

Factors
affecting the rate of concrete sewer pipe corrosion
The
rate at which sound concrete is corroded (once the surface pH <6) varies
from site to site depending on the nature of the local sewer environment. Bruge
1. Impact of H2S
concentration in the sewer headspace
•
As the H2S concentration in the sewer headspace increases so does
the rate of
•
Field studies and literature data suggest that the rate of corrosion increases
with the square root of the HS concentration:

2. Impact of sewer
headspace temperature
•
As the sewer temperature increases the rates of biological activity increases
as as the rates of reaction between the acids produced and concrete.
• The impact of sewer gas temperature (averaged over time) on the rate of corrosion can be described by the following expression:
rate
of corrosion ce (-30,000/Rx(T+273))
3. Impact of Sewer Headspace Humidity
Concrete
is a porous material. As sewer headspace humidity increases more of the pores
within the concrete are filled with water until at 100% humidity all pores are
filled. The activity of bacteria responsible for corrosion increases in an
aqueous environment.
Consequently
as concrete more content increases the rate of corrosion increases.
•
The impact of humidity on the rate of corrosion depends on the distribution of
pore sizes within the concrete and thus will be different for different sewer
pipes.
•
At humidities <85% corrosion is greatly inhibited (the sewer wall feels
“dry”).
•
As humidity increases above 95% the rate of corrosion increases rapidly with
increasing humidity.
•
The impact of humidity can be approximated by the following expression:

Other
factors that can affect the rate of corrosion include:
•
The temperature of the sewer pipe relative to the sewer headspace gas. eongjun
enibool insong of banju
*
The temperature of the sewer pipe can sometimes be different to the sewer
atmosphere inside (especially if the pipe is exposed above ground). This can
have a significant impact on the level of moisture condensing within the
concrete pore structure which in turn can affect the level of corrosion
activity.
The
lining of other sections of the sewer pipe.
Water Supply And Wastewater Engineering: Unit IV: Planning And Design Of Sewerage System : Tag: : Sewerage System - Corrosion in Sewers & Concrete Sewer Pipe
Related Topics
Related Subjects
Water Supply and Wastewater Engineering
CE3303 3rd Semester Civil Dept 2021 Regulation | Tag: 3rd Semester Civil Dept 2021 Regulation
