Water Supply And Wastewater Engineering: Unit IV: Planning And Design Of Sewerage System
Characteristics and Composition of Sewage and their Significance
The quality of sewage can be checked and analysed by studying and testing its physical, chemical and bacteriological characteristics.
CHARACTERISTICS
AND COMPOSITION OF SEWAGE AND THEIR SIGNIFICANCE
The
quality of sewage can be checked and analysed by studying and testing its
physical, chemical and bacteriological characteristics.
Physical
Characteristics of Sewage
(i)
Physical characteristics include:
(i) Colour
(ii) Odour
(iii) Temperature and
(iv) Turbidity,
(i) Colour:
❖ The colour of
sewage can normally be detected by the naked eye, and it indicates the freshness of sewage.
❖ If
the colour is yellowish, grey, (or)
light brown, it indicates fresh sewage.
❖ If
the colour is black (or) dark brown,
it indicates stale and septic sewage.
❖ Industrial waste water imparts colour to
sewage and the color depends on the chemical process in industries.
(ii) Odour:
❖ Fresh
sewage is practically odourless.
❖ After
3 to 4 hours, it becomes stale when all the oxygen present in sewage gets
exhausted.
❖ After
few hours, it starts omitting offensive odours, especially (H,S) hydrogen
zsulphide gas, due to decomposition of sewage.
(iii) Temperature:
❖ Affects
the biological activity of bacteria present in sewage. When temperature is
high, the bacteria will be more active in decomposition of waste in sewage.
❖ When
temperature is high, the solubility of gases in sewage reduces.
❖ The
dissolved oxygen content (D.O) of sewage also gets reduced with high
temperature.
❖ Temperature
affects the viscosity of sewage, which inturn affects the sedimentation process
in sewage treatment.
❖ The
normal temperature of sewage is generally slightly higher than the temperature
of water.
❖ The
average temperature of sewage in India is 20°C, which is ideal for biological
activities.
(iv) Turbidity:
❖ Solids
in suspension cause turbidity.
❖ Sewage
is normally turbid, having floating matter like pieces of paper, match sticks,
greases, vegetable dehris, fruit skins, soaps, etc.
❖ The
turbidity increases as sewage becomes stronger.
❖ It
is an optical light-emitting property.
❖ The
degree of turbidity can be measured and tested by turbidity rods (or) by
turbidity meters (Jackson's turbidity meters, Nephlometric turbidity metres
etc.)
Chemical
Characteristics of Sewage
(i) Total Solids
❖ Sewage
normally contains very small amount of solids (0.05 to 0.1%) in relation tabiat
to the huge quantity of water (99.9%). borgiow It only contains about 0.05 to
0.1 percent (i.e., 500 to 1000 mg/L) of total solids.
Classification
(i) Suspended solids
are solids which remains floating in sewage.
(ii) Dissolved solids
remain dissolved in sewage.
(iii) Colloidal solids
are finely divided solids remaining either in solution (or) in suspension.
(iv) Settleable solids
are solids which settle out, if sewage is allowed to remain undisturbed for a
period of 2 hours.
❖ The
solids can also be categorised as:
(a)
Organic Solids,
(b)
Inorganic Solids.
❖ The
organic matter is about 45% of total solids and remaining 55% is inorganic
matter.
❖ Inorganic
matter consists of minerals, salts, sand, gravel, debris, chlorides, sulphates,
etc. The presence of inorganic solids in sewage is not harmful and requires
simple treatment.
❖ Organic
matter consists of carbohydrate (cellulose, cotton, fibre, starch, sugar,
etc.), Fats and oils received from kitchens and Nitrogeneous compounds like
protein. Organic matter in sewage requires proper treatment before disposal in
water bodies.

Measurements:
a)
Total Solids (S, mg/l) - A known volume of sewage is evaporated and the dry
residue is weighed.

b)
Suspended Solids (S, mg/l)/Non-Filterable Solids.
❖ A
known volume of sewage sample is passed through a glass-fibre filter apparatus
ni (10) no of 1 μm pore size. The dry residue retained on filter is weighed.
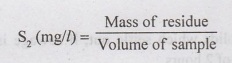
c)
Filterable Solids = Total Solids (S,) – Suspended Solids (S2)
(Dissolved
+ Colloidal Solids).
d)
Total Suspended Solids (S,) may be volatile (or) fixed.
TSS=
Volatile Solids + Fixed Solids
e) Volatile Solids -
Non-filtered residue (of step b) is burnt and ignited at about 550°C in an
electric muffle furnace for about 15 to 20 minutes.
❖ Loss of weight due to ignition will represent
the volatile solids (S, mg/L).
f)
Fixed Solids S5 = S2 – S4 i.e., Suspended
Solids - Volatile Solids.
g) Settlable Solids (S6):
❖ Conical
glass vessel called Imhoff cone is used.
❖ The
capacity of the cone is 1 litre and it is graduated upto about 50 ml.
❖ Sewage
is allowed to stand in this imhoff cone for a period of 2 hours and the
quantity of solids settled in the bottom of the cone can be directly read out.
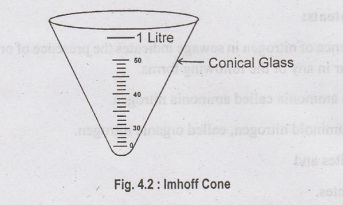
(ii) pH Value:
❖ pH
value indicates negative log of hydrogen ion concentration
pH
= -log H+ (or) H+ = (10)-pH
pH
<7-acidic range
pH
> 7 - alkaline range.
❖ The
fresh sewage is generally alkaline in nature (pH more than
❖ But
as time passes, sewage turns acidic and its pH tends to fall due to production
of acids by bacterial action.
❖ The
pH value can be measured quickly and automatically with the help of
potentiometer, which measures the electrical potential exerted by the hydrogen
$2015 ions.
❖ The
efficiency of sewage treatment depends on pH.
❖ If
pH of sewage is low, lime is added to create alkaline condition.
(iii) Chloride
Contents:
❖ Chlorides
are generally found in domestic sewage, and are derived from the kitchen
wastes, urinary discharges, feces etc.
❖ Large
amount of chlorides may enter from industries like ice cream plants, meat
salting industries.
❖ Chloride
in sewage may also be due to infiltration of sea water (NaCl).
❖ The
normal chloride content of domestic sewage is 120 mg/l.
❖ The
chloride content can be measured by titrating the waste water with standard
silver nitrate AgNO, solution using potassium chromate as indicator.
(iv) Nitrogen
Contents:
❖ The
presence of nitrogen in sewage indicates the presence of organic matter, and it
may occur in any of the following forms.
a)
Free ammonia called ammonia nitrogen.
b)
Albuminoid nitrogen, called organic nitrogen.
c)
Nitrites and
d)
Nitrates.
NH3
↑ +H20 NH4++OH-
If
further oxidation occurs as
NH4++
202 → NO3-+2H++H20
❖ The
sewage treatment is done using microorganisms which decompose the organic waste
into stable compounds.
❖
The presence of nitrogen in various forms is indicative of the stages of
decomposition and level of treatment.
❖ The
free ammonia indicates the age of waste water and the very first stage of nogobert
decomposition of organic matter.
❖ Albuminoid
nitrogen indicates quantity of nitrogen in sewage before the decomposition of
organic matter is started.
❖ The
nitrites indicate the presence of partly decomposed (not fully oxidised)
organic matter viz., treatment is in progress.
❖ The
nitrates indicate the presence of fully oxidised organic matter viz., treatment
is complete.
a) Free Ammonia
❖ Indicates
age of waste water.
❖ Its
presence indicates stale or old sewage.
❖ Indicates
very first stage of decomposition of organic matter in sewage.
❖ Measured
by distillation process.
b) Albuminoid
Nitrogen
❖ Indicates
nitrogen content in waste water before decomposition of organic matter has
started.
❖ Measure
of undecomposed Nitrogen.
❖ Measured
by treating sample with alkaline solution of potassium permanganate.
c) Nitrites Nitrogen
❖ Presence of nitrites
indicates presence of partly decomposed organic matter.
❖ Indicates treatment is still incomplete and sewage is stale.
❖ Measured by Colourimetric Method [Colour Matching Method].
[Adding
Sulphonilic Acid and Naphthamine]
d) Nitrate Nitrogen
❖ Presence of nitrate
indicates presence of fully oxidised organic matter.
❖ Stable form of
nitrogenous matter.
❖ Indicates
oxidised/treated wastewater.
❖ Measured by
colourimeteric method (colour matching methods) and compared with standard
colours. (Adding Phenol di-sulphonic acid and potassium hydroxide).
* Blue Baby Disease
(Methaemoglobinemia) - affects Infants
❖ If the nitrate content
is above 45 ppm in water, it may cause nitrate poisoning in infants.
❖ This happens because
there exists lower acidity in the intestines of the infants, which permits the
growth of nitrate-reducing bacteria, which converts nitrates to nitrites.
❖ The nitrites prove very
harmful because they have greater affinity for haemoglobin than oxygen and thus
completely reduce oxygen level in blood causing suffocation, turning the body
of infants to blue colour.
(v) Presence of Fats,
Oils and Greases:
❖ Greases, fats and oils
are derived in sewage from the dischages of animals, vegetable matter from
garages, hotels, restaurants and industries etc.
❖ They interfere with
sewage treatment and they form scum on the top of sedimentation tanks and clog
the voids of the filtering media.
❖ They are not
decomposible by bacterial action and therefore should be removed from sewage.
❖ Measured by evaporating
sewage sample, residual solids left after evaporation are mixed with ether
(hexane) and again evaporated, leaving behind the fats and greases as a
residue, which can be weighed.
(vi) Sulphides,
Sulphates and Hydrogen Sulphide Gas:
❖ Formed due to the
decomposition of various sulphur containing substances in sewage.
❖ This decomposition also
leads to evolution of hydrogen sulphide (H,S) gas, causing bad smells odours
and corrodes the sewer pipes.

❖ Formation of sulphides
hinders the process of sludge digestion.
(vii) Dissolved
Oxygen (D.O):
❖ D.O. is the oxygen
present in dissolved state in waste water, which prevents noxious odours.
❖ D.O test performed on
sewage before treatment helps in indicating the condition of sewage and decides
selection of treatment methods.
❖ Fresh sewage contains
some dissolved oxygen, which is soon depeleted by aerobic decomposition.
❖ Presence of D.O. in
treated sewage indicates oxidation during treatment stages.
❖ Treated sewage effluent
should have atleast 4 ppm D.O. in it, otherwise it will affect the aquatic life
when sewage is discharged into water bodies.
❖ The dissolved oxygen in
fresh sewage depend upon temperature.
❖ If the temperature of
the sewage is more, the D.O content will be less.
❖ The D.O content of
sewage is determined by Winkler's method which is an oxidation-reduction
process, wherein iodine liberated is equivalent to the D.O.
(viii) Oxygen Demand:
❖ Oxygen required for
oxidation of both inorganic as well as organic matter.
BOD
- Biochemical Oxygen Demand.
COD
- Chemical Oxygen Demand.
TOD
- Total Oxygen Demand.
ThOC
- Theoretical Oxygen Demand.
TOC
- Total Organic Carbon.
(ix) Chemical Oxygen Demand (COD):
❖ Measure of Oxygen (O2)
required to oxidise organic matter in sewage into CO2, H2O
and oxidised species.
❖ Chemicals are used to
oxidise both biologically active and inactive organic matter in sewage.
❖ COD test - Oxidation
using potassium dichromate.
❖ A known quantity of
waste water is mixed with known quantity of potassium dichromate and the
mixture is heated. The organic matter is oxidised by K2Cr2O7
(in the presence of H2SO4). The resulting solution of K2Cr2O7
is titrated with Ferrous Ammonium Sulphate (FAS) and the oxygen used in
oxidising the waste water is determined. This is called Chemical Oxygen Demand
(COD).

(x) Biological Oxygen
Demand (BOD):
❖ Measure of oxygen required
to oxidise biologically active organic matter in sewage by microorganisms.

* Biologically Active →
The organic matter which can be oxidised by microorganisms is called
biologically active (under aerobic conditions at standard temperature).
* Significance of BOD:
BOD
of waste water decides the following: noir
(1)
Quantity of O2 required for biological stabilization of organic
matter in sewage.
(2)
Size of treatment facilities.
(3)
Measure of efficiency of treatment.
(4)
Dilution required for disposal of waste water.
Aerobic
bacteria utilises the organic matter and oxygen in sewage and starts
multiplying, the bacterial count increases which causes faster decomposition of
organic matter in sewage. The oxygen
consumed by the bacteria for decomposition of organic matter in sewage is BOD.
Practically
it is not feasible to determine the ultimate BOD.
Hence
BOD at 20°C during 5 days is taken which is 68% of the total BOD.
BOD5d
=0.68 BOD
BOD5
= 5 day BOD
BOD
u = Ultimate BOD.
* BOD5/BOD Test:
(Dilution Method)
❖
Known volume of sewage sample is diluted with known volume of aerated pure
water.
❖ Diluted
sample is incubated for 5 days at 20°C.
❖ The
D.O of the diluted sample before and after incubation period is measured.
❖ The
difference between the initial D.O value and final D.O value indicates the
oxygen consumed by microorganisms in 5 days (causing aerobic decomposition).
BOD/BOD,
= D.O consumed by diluted sample x
[Volume
of diluted sample /Volume of undiluted sample]
[DF
= 1% = 1 ml. of sewage diluted to make 100 ml, hence multiplied by 100].
Normally, 300 ml BOD test bottles are used
If
4 ml of sewage sample is taken - Incubation at 20°C-5 days.
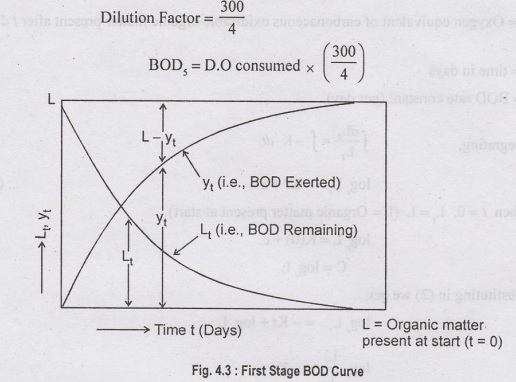
The
BOD rate at any time depends on temperature and also on the amount and nature
of organic matter present in sewage at that time.
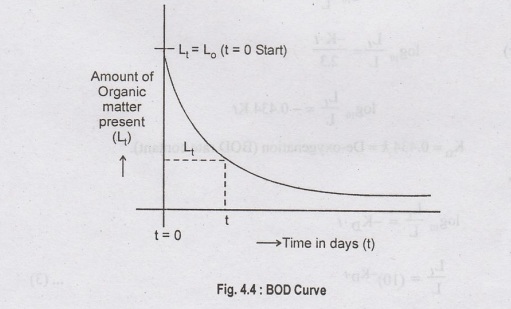
At
a certain temperature, the rate of deoxygenation is assumed to be directly
proportional to the amount of the organic matter present in sewage at that time
i.e.,

L1
= Oxygen equivalent of carbonaceous oxidisable organic matter present after t
days
(mg/l)
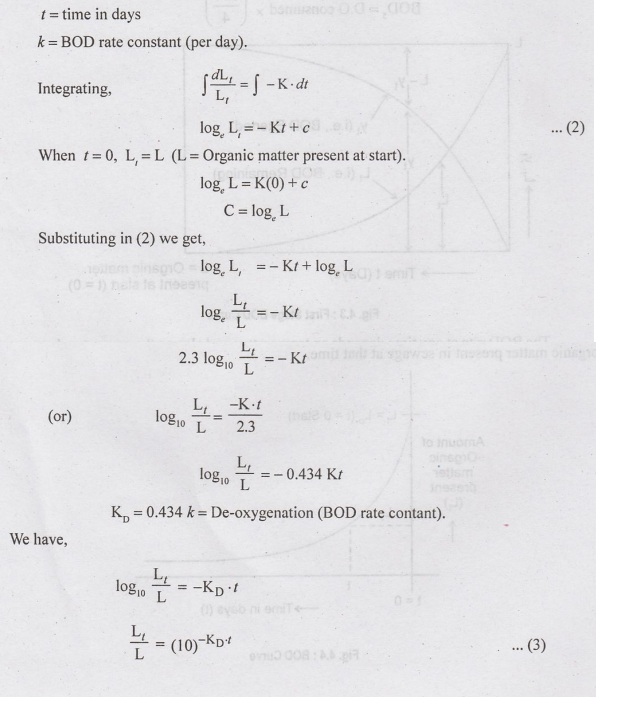
Now
L is the organic matter present at the start of BOD reaction and L, is the
organic matter left after t days, which means that during t days, the quantity
of organic matter oxidised = L-Li
If
Y, represent the total amount of organic matter oxidised in t days, then we
have,

Taking
L out of bracket on R.H.S.

Substituting
equation (3),

Yt is the oxygen absorbed in t days.
Ultimate
BOD (Yu )i.e., When t= ∞ days.
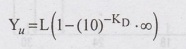
Yu
=L = Ultimate BOD = Organic matter in sewage.
The
ultimate BOD (Yu)
is equal to the oxygen equivalent organic matter present in sewage (L).
BOD rate constant (KD)
The
value of KD determines the speed of the BOD reaction.
❖BOD rate
constant is temperature dependent.
❖ KD
is higher at higher temperature and rate of oxidation will be faster.
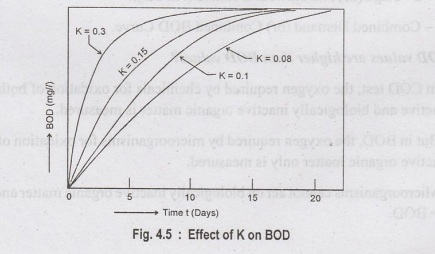

The
oxygen demand during the first 20 days is due to oxidation of organic matter in
sewage and is called Carbonaceous BOD or
first stage demand (CBOD). The latter oxygen demand occurs due to
biological oxidation of ammonia and is called as second stage BOD or nitrogenous BOD (NBOD).
OAB
- 1st stage (or) Carbonaceous Stage (CBOD).
AC
- 2nd stage (or) Nitrogenous Demand (NBOD).
OAC
- Combined Demand (or) Combined BOD Curve.
*
Why COD values are higher than BOD values?
❖ In
COD test, the oxygen required by chemicals for oxidation of both biologically
active and biologically inactive organic matter is measured.
❖ But
in BOD, the oxygen required by microorganisms for oxidation of biologically
active organic matter only is measured.
❖ Microorganisms
cannot act on biologically inactive organic matter and hence COD > BOD.
❖ BOD to COD ratio
BODu
BOD = Measure of Biodegradable organics.
COD
= Measure of both Biodegradable + Non-Biodegradable organics.
Since
COD > BOD => BODu/ COD ratio always < 1
If
ratio = 0.92 to 1.0 = Waste water is fully biodegradable.
BOD5
/COD = 0.63 to 0.68 →→ Indicates that Wastewater can be biologically treated →
Indicates minimum quantity of non-biodegradable organic matter.
...
BOD5= 0.68 BODu
(xi) Total Organic Carbon (TOC) and
Theoretical Oxygen Demand (Th.OD)
❖ Carbon
is the primary constituent of organic matter. TOC therefore gives a measure of
organic matter in sewage.
❖ Organic
matter is expressed as carbon content.
❖ Chemical
formula (Cn Hn On Nn) and
concentration of compounds present in wastewater enables us to calculate
theoretically the carbon content in wastewater per litre of solution.
(Cn
Hn OnNn ) → Carbon, Hydrogen, Oxygen,
Nitrogen
For example:

Water Supply And Wastewater Engineering: Unit IV: Planning And Design Of Sewerage System : Tag: : - Characteristics and Composition of Sewage and their Significance
Related Topics
Related Subjects
Water Supply and Wastewater Engineering
CE3303 3rd Semester Civil Dept 2021 Regulation | Tag: 3rd Semester Civil Dept 2021 Regulation
